1.
Abstract-Objective: This work is aimed at expanding the field of application of natural biomass for the treatment of dye waste effluents. It is equally aimed at determining the dependency or otherwise of the effect of flow rate and bed height on the fixed bed adsorption of methylene blue dye onto Sphagnum cymbifolium (moss). Methods: The biomass was characterized by scanning electron microscopy (SEM) for the determination of the morphology of the biomass. The screened biomass samples were characterized at 1000X magnification, 500X magnification and 250X magnification respectively for their surface morphologies. This was done using a scanning electron microscope (FEI-inspect/OXFORD INSTRUMENTS-X-MAX), which was equipped with an energy dispersive x-ray (EDAX) spectrophotometer employed for the elemental composition analyses. It was equally characterized with Fourier transformed infrared spectroscopy (FTIR) before and after adsorption to ascertain the functional groups responsible for the adsorption. This was done using a Fourier Transformed Infrared (FTIR) spectrophotometer (Perkin Elmer, England) in the wavelength range of 350-4000nm.
Results: Results for the biomass morphology through the scanning electron microscopy (SEM) showed the presence of some pores. These pores represent sites where dye molecules could be trapped in the course of adsorption. The results from the Fourier Transformed Infrared Spectroscopy (FTIR) before adsorption revealed the presence of five functional groups.
The functional groups include O-H or N-H, C-H, C?N or C?C, C=O, or C=C and benzene. However, after the adsorption, it was found that the functional groups that were responsible include C-H, C?H and C?C. Within the level of experimental consideration, it was found that the rate of adsorption was dependent on flow rate and bed height. An increase in flow rate and bed height led to a corresponding increase in the value of q e . Conclusion: From the results obtained, it is seen that methylene blue dye can absorb on to Sphagnum cymbifolium (moss) through the fixed bed process. Also, within the limit of experimental consideration, that the adsorption of methylene blue dye onto Sphagnum cymbifolium (moss) through the fixed bed technique is flow rate and bed height dependent. In each of the analyses, three different experiments were performed and the mean values reported with their standard deviations.
2. INTRODUCTION
any industries such as plastic, dyestuffs, textiles and inks, use dyes to color their products, and also consume substantial volumes of water. Due to their good solubility, synthetic dyes are common water pollutants. The presence of very small amounts of dyes from waste waters before it is discharged into the environment. Adsorption techniques are proved to be an effective and attractive process for the removal of nonbiodegradable pollutants (including dyes) from waste waters [1]. Most commercial systems use water because it has excellent adsorption ability. But, its widespread use is limited due to high running cost. Due to that, many low cost adsorbents, and waste materials from industry and agriculture have been proposed by several workers []. These materials do not require any expensive additional pre-treatment step and could be used as adsorbents for the removal of dyes from solution.
Some researchers reported the use of plant leaf biomass to adsorb heavy metals from solutions [2]. Resh water algae Pithophore sp; was studied by Kumar in finding out its bio-sorption properties on to malachite green (a cationic azo dye) [3]. This work is carried out with the view of expanding the field of application of natural biomass for the treatment of dye waste water through the fixed of application of natural biomass for the treatment for the treatment of dye waste water through the fixed bed technique. Also, it is aimed at determining the impact of flow rate and bed height on the fixed bed adsorption of methylene blue dye on to Sphagnum cymbifolium (moss). Since such as in-depth consideration has not been done on this biomass, the information obtained will add to the expansion of knowledge in this area.
3. II.
4. MATERIALS AND METHODS
The methylene blue dye, calcium chloride, distilled water and other necessary reagents used in this work were obtained from qualikem laboratory, owerri, Nigeria. The Sphagnum cymbifolium (moss) used was obtained from Ikorodu area in Lagos, Nigeria which is located within the following co-ordinates 6.6194°N and 3.5105°E. This sample was identified at the department of Crop science at the Federal university of technology, M lobal Journal of Researches in Engineering ( ) Volume Xx XI Issue II Version I J Owerri, Nigeria with the voucher specimen number of FUT/CR/005/17. The biomass was washed severally with distilled water to remove any dirt from it. The washed biomass was air dried for ten days until a constant weight was obtained. The biomass was grinded with a new sonic domestic blender to avoid any form of contamination. It was further screened using 600-800 micron sized sieves and stored in air tight containers ready for adsorption.
This methods and techniques employed in these analyses are the standard methods which have been used by other researchers [4].
5. III. CHARACTERIZATION OF THE BIO-SORBENT
The surface structure and morphology of the Sphagnum cymbifolium (moss) was characterized at 1000X magnification, 500X magnification, and 250X magnification, respectively. This was done using scanning electron microscopy (SEM) (FEI-Inspect oxford instrument-x-max), which was equipped with an energy dispersive x-ray (EDAX) spectrophotometer employed for elemental composition analysis.
The biomass sample was further characterized for their fundamental functional groups before and after adsorption experiment using Fourier Transformed Infrared (FTIR) spectrophotometer (Perkin-Elmer, England) in the wavelength range of 350-4000nm using KBr powder and fluka library for data interpretation.
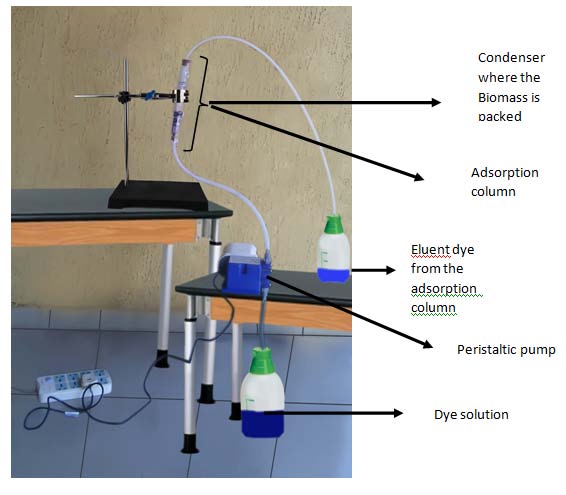
6. a) The Fixed Bed Set Up
The fixed bed was set up by packing wire gauze, glass wool, glass beads, glass wool, biomass and glass wool in that order in a graduated condenser. Then a dye solution of a known concentration and pH pressurized from down to top where a known amount of the bio-sorbent is placed with a peristaltic pump (CHEM-TECH model X030-XB-AAAA365, China). Subsequently, a sample was collected for u.v analysis in a u.v spectrophotometer (CAMPEC M 106 Model, England) by monitoring the absorbance already determined for methylene blue dye at 600nm. The variables investigated include the effect of flow rate and bed height.
7. b) Effect of Flow Rate on Adsorption
Experiments were performed at different flow rates of 2nm 3 /s, 30m 3 /s and 40m 3 /s respectively, while keeping constant a bed height of 1x10 -2 m, 40mg biomass dose, 90mg/L and a pH of 4. The dye solution was subjected to pass through the column already prepared using the peristaltic pump. The samples collected were subjected to u.v analysis for absorbance values were converted to concentration by the use of Beer Lambert law. Similar experiments were carried out in triplicates and the mean values and standard deviation reported.
8. c) Effect of Bed Height on Adsorption
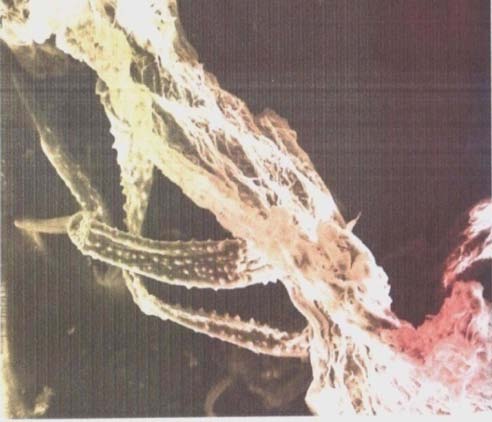
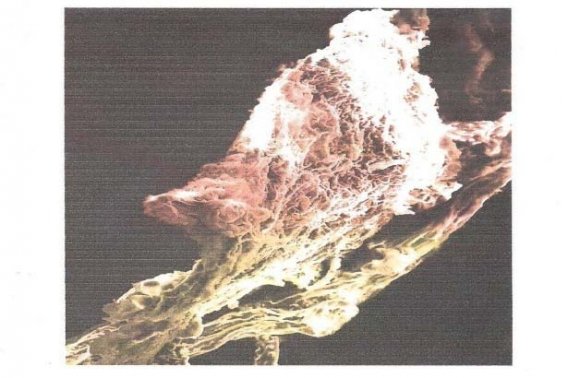
Experiments were conducted at different bed height of 4x10 -2 m, 5x10 -2 m and 6x10 -2 m while keeping constant a flow rate 10m 3 /s. 90mg/L dye solution, pH of 4 which is the pH of maximum adsorption for methylene blue dye. The dye solution was subjected to pass through the column already prepared using the peristaltic pump. The samples collected were subjected to u.v analysis for absorbance measurements at 600nm. Subsequently, the absorbance values were converted to concentration by the use of Beer Lambert's law. Similar experiments were carried out in triplicates and mean values and standard deviations reported. The SEM micrographs of Sphagnum cymbifolium (moss) showed the presence of unevenly dispersed cavities on the surface of the biomass. These cavities provide sites where the molecules of the dye could be trapped in the course of the adsorption. The SEM micrographs of (X250), (X500) and (X1000) magnifications are shown in fig. 2, 3 and 4 respectively.
9. NOTE: The amount of dye adsorbed per gram biomass
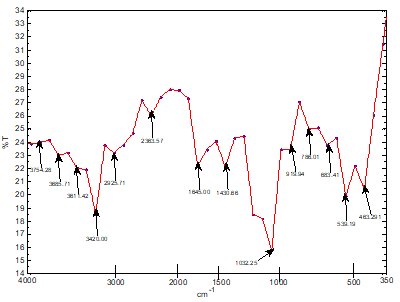
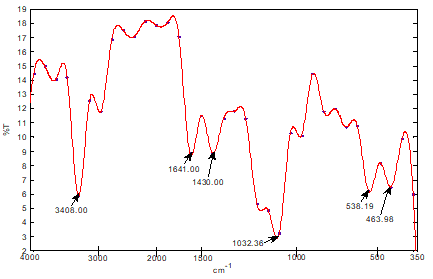
Similar cavities have been discovered by other researchers [5]. The FTIR Spectrum of Sphagnum cymbifolium (moss)) after adsorption as shown in fig. 6 above was used to ascertain the functional groups that were responsible for the adsorption. After the adsorption, there were some bond displacement of the original peaks indicating the functional groups that were responsible for the adsorption reaction. The displacements occurred at 2925.71nm and 2363.51nm which corresponds to C-H, C?N and C?C functional groups. Although, the intensity of the peaks greatly decreased after the adsorption, the functional groups on the biomass did not disappear totally during the biomass characterization after the adsorption. As could be seen from fig. 7, an increase in flow rate caused a corresponding increase in the q e values within the range of experimental consideration. A similar effect was reported by other researchers [6]. This could be due to increase in the force of attraction between the dye solution and the biomass surface area. Figure 8 shows the effect of bed height on the quantity of the methylene blue dye adsorbed onto the biomass (q e ). The q e values for the biomass increased with a corresponding increase in the bed height within the range of experimental consideration. The result indicates that, the longer the bed height, the higher the q e values. A similar situation has been reported in similar investigations [7]. This could be attributed to the longer time of interactions between the biomass and the dye solution.
V.
10. CONCLUSION
The findings of this research vividly reveal that methylene blue dye can be adsorbed on to Sphagnum cymbifolium (moss) biomass through the fixed bed process. Additionally, the two variables, flow rate and bed height can impact the adsorption properties of methylene blue dye on to Sphagnum cymbifolium (moss).