1. H
Currently, there are several effective techniques for removal of heavy metal ions: chemical precipitation (Fu et al., 2011), ion exchange (Zewail et al., 2015), adsorption (Kumar et al., 2016), membrane filtration (Yurekli et al., 2017), electrodialysis (Nemati et al., 2017), reverse osmosis (Li et al., 2017), etc. Among them, the adsorption seems to be the most attractive technique due to its recovery merit, low cost, easy handling and simple design requirement (Kurniawan et al., 2006). However, it is difficult to remove the total metal ions by the only adsorption because of the physicochemical limitation of adsorptive rate particularly at the low concentration range of target metals. In contrast, a coupling method of the adsorption and the photoelectrodeposition is a more effective technique for the removing heavy metal ions at low concentrations due to utilizing irreversible photocatalytic redox reaction. Commonly, semiconductor photocatalysts have been used as an adsorbent to occur the photoelectrodeposition (Kobayashi et al., 2017;Nozaki et al., 2018).
The wide band gap semiconductors such as TiO 2 , WO 3 , SnO 2 , and CeO 2 have mostly been used as a photocatalyst due to their thermal and chemical stability, low cost and environmental-friendly (Ji et al., 2009;Zhang et al., 2018). Among them, the CeO 2 has received much attention for the unique characteristics. The CeO 2 possesses large oxygen storage and releasing ability derived from the redox cycle between Ce 3+ and Ce 4+ , and the CeO 2 is often utilized for the catalytic oxidation reaction. The CeO 2 therefore plays an important role as a photocatalyst for photo-oxidation and photo-reduction against adsorbed substances (Ke et al., 2014;Qiang et al., 2015). Furthermore, the CeO 2 shows a strong UV absorption and does not cause the photolysis during the photocatalysis (Magesh et al., 2009), while only CeO 2 nanoparticles show low adsorption capacity of heavy metal ions. In the pioneering reports, improvement on the adsorption capacity was attempted by loading heterogeneous semiconductor materials possessing the high adsorption capacity and the high carrier conductivity for the surface photoelectrodeposition such as Gd 2 O 3 (Ayawanna et al., 2015), La 2 O 3 (Ayawanna et al., 2017), ZnO (Nozaki et al., 2018), etc., on the CeO 2 as an auxiliary catalyst. However, these metal oxides easily eluted instead of the adsorption of heavy metal ions as an ion-exchange manner because of their higher ionization tendency (Ayawanna et al., 2015;Ayawanna et al., 2017;Nozaki et al., 2018). Thus, an excellent loading material possessing high adsorption capacity, high carrier conductivity and chemical stability was desired.
Tin-oxide compounds (SnO x ) are focused in this study. The SnO x has prominent superiorities such as high abundance, non-toxicity, low ionization tendency, high adsorption capacity of heavy metal ions, and fast carrier mobility between band structure and surface (Hamdi et al., 2017;Dey, 2018). Thus, the SnO x offers many technological applications such as photocatalysts (Zhao et al., 2018), solid-state gas sensors, and adsorbents for the removal of heavy metal ions (Kumar et al., 2016;Dey, 2018). Motivated by these factors, the SnO x was loaded on the CeO 2 surface to prepare a new photocatalyst for the adsorption and the photoelectrodeposition of heavy metal ions.
The primary purpose of this study was to synthesize the SnO x /CeO 2 photocatalysts and evaluate its removal ability of heavy metal ions especially lead ion (Pb 2+ ). In addition, it was also a major purpose to obtain further knowledge of the photoelectrodeposition phenomenon which detailed mechanism has not been elucidated yet.
2. II.
3. Materials and Methods
4. a) Chemicals and reagents
Ce(NO 3 ) O, and L(+)-ascorbic acid were all purchased from Wako Pure Chemical Industries Co., Ltd. 4-(2-pyridylazo)-resorcinol was purchased from Dojindo Molecular Technologies Co., Ltd. 2,3diaminonaphthalene and 1,5-diphenylcarbazide were purchased from Tokyo Chemical Industry Co., Ltd. All other reagents were at least of reagent grade and used without further purification. Milli-Q water was used for the preparation of all aqueous solutions.
5. b) Synthesis of SnO x /CeO 2
CeO 2 was prepared by the polymerized complex method. Firstly, 10.0 g of citric acid was dissolved in 11.8 g of ethylene glycol with heating at 50 ?C for 30 min under stirring at 600 rpm by a hot magnetic stirrer. Then, 5.0 g of Ce(NO 3 ) 3 ? 6H 2 O were added to the solution and heated at 200 ?C for 1 h under stirring at 600 rpm. Finally, the obtained precursor gel was calcined at 350 ?C for 1 h and then at 1000 ?C for 5 h with a heating rate of 10 ?C/min (Kakihana et al., 1992). SnO x /CeO 2 were prepared by the impregnation method. The prepared nanoparticles the abovewere impregnated in an aqueous solution of SnCl 2 ? 2H 2 O, and the solution was heated at 150 ?C under stirring at 400 rpm. After the evaporation of water, the obtained sample was calcined at 650 ?C for 3 h with a heating rate of 10 ?C/min (Murayama et al., 2017).
6. c) Characterization
The crystal phase and structure of the obtained photocatalysts were analyzed by the powder X-ray diffraction (XRD, D8 ADVANCE, Bruker AXS) with CuK? radiation. The chemical states and composition of the photocatalyst surface were identified using the X-ray photoelectron spectroscopy (XPS, PHI X-tool, ULVAC-PHI) operated with AlK ? were calibrated by fixing the C 1s peak of the surface carbonaceous contaminants at 284.8 eV. The Brunauer-Emmett-Teller (BET) specific surface area of the photocatalysts were elucidated using a BET surface area analyzer (Flowsorb ?, d) Removal of Pb 2+ An aqueous solution including 20.0 mg/L Pb 2+ was prepared. Then, 0.130 g or 0.200 g of the SnO x /CeO 2 photocatalyst particles were added into a 20.0 mL of the prepared aqueous solution in a 30 mL beaker. The suspension was stirred at 500 rpm for 150 min by a magnetic stirrer and was also being irradiated with a UV-LED light (NCSU033B, 7.8 mW/cm 2 , 360-370 nm, NICHIA). Another similar experimental set was carried out on the same suspension under stirring in dark condition. After the experiments, the reaction suspension was sampled and filtered, and the pH of the filtrate was measured using a pH meter (PH-201, SAGA). The residual concentrations of Pb 2+ in the filtrate was measured by the colorimetry with 4-(2-pyridylazo)resorcinol at 520 nm (Kocy?a et al., 2015). The effect of different initial pH on Pb 2+ removal was examined. The pH was adjusted using either 0.10 M HCl or 0.10 M NaOH solutions.
In the time-course removal experiments of Pb 2+ , 1.000 g of the SnO x /CeO 2 photocatalysts were added into a 100.0 mL of the aqueous solution including 20.0 mg/L Pb 2+ in a 100 mL beaker due to sampling 5.0 mL of the reaction suspension at designated times (10, 30, 60, 105 and 150 min). The obtained data were fitted to the pseudo first-order model (Equation ( 1)) and pseudo second-order model (Equation ( 2)) (Lagergren et al., 1898;Ho et al., 1999):
?? ?? = ?? ?? (1 ? ??????(??? 1 ??))(1)?? ?? = ?? ?? 2 ?? 2 ?? (1+?? ?? ?? 2 ??)(2)Adsorption and Photoelectrodeposition of Heavy Metal Ions from Wastewater using SnO x(1<X<2) /CeO 2 Photocatalysts CeO 2 radiation. The Chemical Shifts Shimadzu). The morphology and microstructure of the photocatalysts were elucidated using a field emission scanning electron microscopy (FE-SEM, Quanta 250 FEG, FEI). Determination of the point of zero charge (pH PZC ) of the photocatalyst surface was employed according to the Park and Regalbuto's procedure (Park et al., 1995). Where q t (mg/g) is the adsorption capacity at time t (min), q m (mg/g) is the maximum adsorption capacity, k 1 (/min) and k 2 (g/mg/min) are the equilibrium rate constant of pseudo first-order adsorption and pseudo second-order adsorption, respectively.
7. Global
8. e) Removal of other heavy metal ions
In the removal experiments of heavy metal ions other than Pb 2+ , an aqueous solution including 20.0 mg/L Cu 2+ , Cd 2+ , Hg 2+ , As 3+ , Se 4+ , and Cr 6+ was prepared with adjusting pH to about 5~6 by using 0.10 M HCl or 0.10 M NaOH solutions. After each removal experiments, the residual concentrations of Cu 2+ , Cd 2+ , and Hg 2+ in the filtrate were measured by the inductivity coupled plasma atomic emission spectroscopy (ICP-AES, PS7800, and HITACHI). The residual concentrations of As 3+ (As 5+ ), Se 4+ (Se 6+ ), and Cr 6+ in the filtrate were measured by the redox treatment and colorimetry with ammonium molybdate and ascorbic acid at 840 nm (Lenoble et al., 2003)
9. Results and Discussion
10. a) Characterization of the obtained photocatalysts
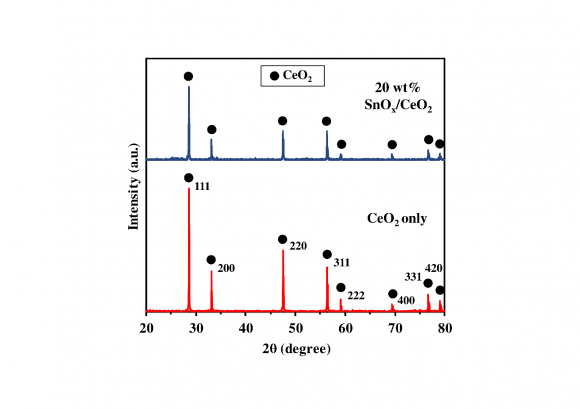
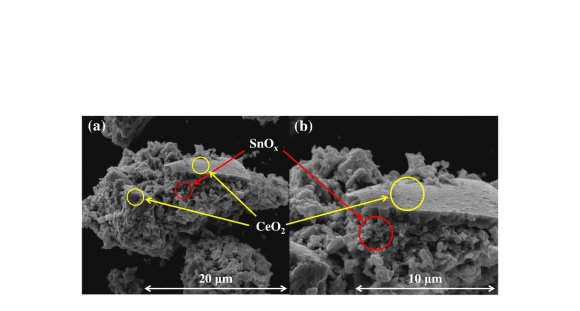
The XRD analysis was implemented to investigate the crystal phase and structure of the obtained photocatalysts. Fig. 1 shows the XRD patterns of CeO 2 and 20 wt% SnO x /CeO 2 . In the XRD pattern of CeO 2 only, the diffraction peaks were in good agreement with those of the cubic fluorite-structured CeO 2 crystal phase (ICDD PDF# 00-004-0593), and the sharpness of the peaks demonstrated the obtained CeO 2 particles possessing high crystallinity. In the XRD pattern of the 20 wt% SnO x /CeO 2 composites, no specific peaks were observed except for those of the CeO 2 phase. It was indicated that the loaded SnO x existing as amorphous and highly dispersed small nanoparticles on the CeO 2 surface.
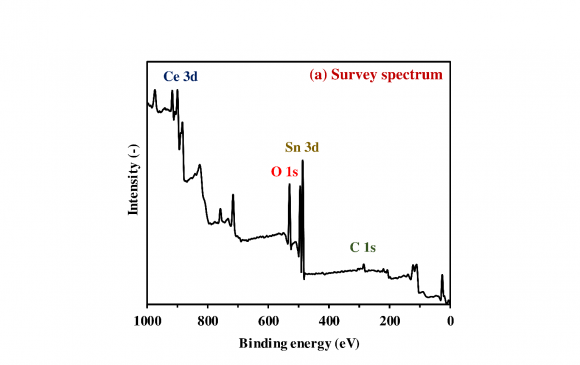
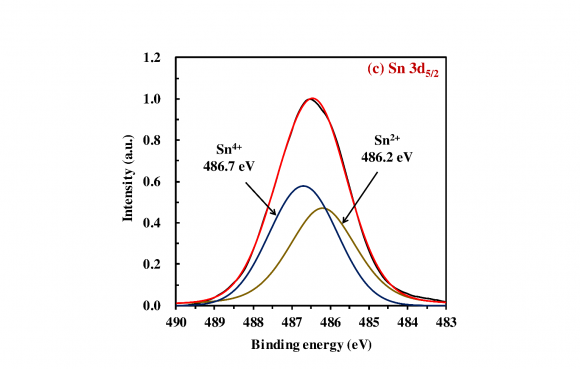
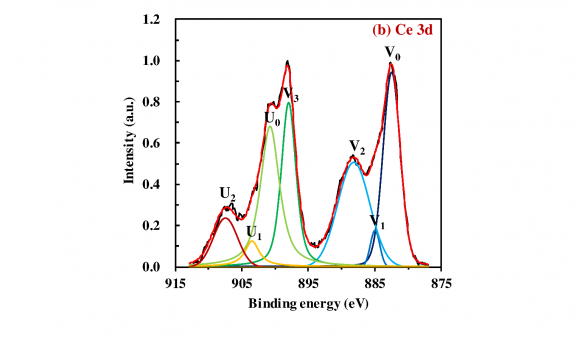
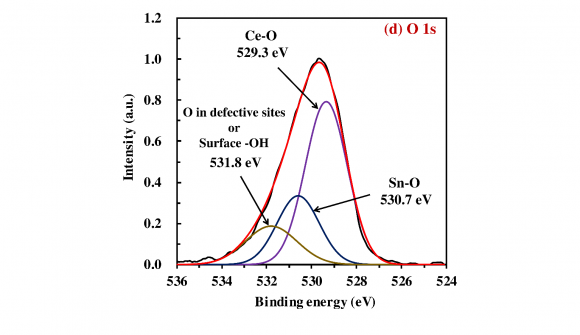
The XPS analysis was implemented to investigate the surface chemical states and composition of the obtained photocatalysts. Fig. 2a presents the survey spectrum of the 20 wt% SnO x /CeO 2 . The appearance of peaks which are attributed to the Ce 3d, Sn 3d, and O 1 s implied the existence of the Ce, Sn, and O elements on the surface of the obtained photocatalysts, and no specific peaks which are attributed to the other elements were observed except the C 1s peak. To investigate the detailed chemical state of the Ce, Sn, and O elements, the high-resolution XPS spectra of the Ce 3d, Sn 3d 5/2 , and O 1s were measured (Fig. 2b-d). In the spectrum of the Ce 3d (Fig. 2b), the de-convoluted peaks labeled U and V are corresponding to Ce 3d 3/2 states and Ce 3d 5/2 states, respectively. According to the literature, U 0 , U 2 , V 0 , V 2 , and V 3 are all the characteristic peaks of Ce 4+ , and U 1 and V 1 are attributed to the photoemission from Ce 3+ (Lu et al., 2016). The measured peaks therefore demonstrated the primary existence of a CeO 2 phase with a small Ce 2 O 3 phase on the surface of the 20 wt% SnO x /CeO 2 . In the spectrum of the Sn 3d 5/2 (Fig. 2c), the de-convoluted Sn 3d 5/2 spectra showed two adjacent peaks located at 486.2 and 486.7 eV, corresponding to the Sn 2+ and Sn 4+ , respectively (Babu et al., 2018). The coexistence of these two peaks indicated the presence of the SnO x(1<x<2) on the photocatalyst surface. According to this coexisting of the two states and the XRD pattern is shown in Fig. 1, it was suggested that the amorphous state SnO x(1<x<2) nanoparticles loading on the CeO 2 with the high dispersion states. In the spectrum of the O 1s (Fig. 2d), three de-convoluted peaks located at 529.3, 530.7, and 531.8 eV were observed. The first two peaks at 529.3 and 530.7 eV are probably associated with the lattice oxygen (O 2-) in the stronger Ce-O band and Sn-O band, respectively. However, since the peaks of the O 1s of Ce 2 O 3 , CeO 2 , SnO, and SnO 2 are very close, assignment of these peaks was puzzling. The last peak at a higher binding energy of 531.8 eV was attributed to the oxygen in defective sites or surface hydroxyl groups (Nagasawa et al., 1999;Ke et al., 2014). the presence of this peak indicated that much oxygen defects were generated on the SnO x surface with the amorphous nature and H 2 O molecules then entered the oxygen defects or coordinated to the Sn 4+ exposed on the surface. Finally, the hydroxyl groups were formed at the surface. According to the literature, the surface hydroxyl groups can be the functional groups for the adsorption of heavy metal ions on the surface of metal oxides (Wu et al., 2018).
11. b) Removal of Pb 2+
Although the amorphous SnO x has rich defect structures, it probably has the high mobility of the carrier due to the characteristics of the tin oxide compounds To optimize amounts of the SnO x loading on the CeO 2 , the relationship between amounts of the SnO x loading and amounts of Pb 2+ removal was examined as shown in Fig. 4. volumes of Pb 2+ removal by the ADS and ADS + PED linearly increased along the increase of the amounts of the loaded SnO x , up to 20 wt%. It was suggested that the SnO x loaded on the CeO 2 was efficient for the improvement of a removal ability of Pb 2+ because the available adsorption sites of Pb 2+ are easily formed on the surface of the SnO x . In the case of loading SnO x more than 20 wt%, the amounts of the PED removal decreased than that of 20 wt% SnO x /CeO 2 . This result is because the existence ratio of the CeO 2 photocatalyst decreased along with the increase of the SnO x latio, and several photocatalytic active sites were physically covered by the excess SnO x particles. Thus, the optimum amounts of the loaded SnO x were 20 wt%. (Dey, 2018). Also, according to the ICP-AES analysis, the ion-exchange elution of Sn ions and Ce ions were not detected in the removal process of Pb 2+ . The promising usefulness of the SnO x as a loading material was therefore reconfirmed. Fig. 5 shows the time-course removal of Pb 2+ by the 20 wt% SnO x /CeO 2 . Also, Table 1 shows the analyses of the Pb 2+ adsorption kinetics of the 20 wt% SnO x /CeO 2 by the pseudo first-order and the pseudo second-order models. As shown in Fig. 5, the adsorption did not reach equilibrium within 150 minutes, and for that, the pseudo second-order model was more suitable than the pseudo first-order model for describing the adsorption process. This result was probably explained by the adsorption of Pb 2+ on the rough surface of the 20 wt% SnO x /CeO 2 and the influence of the diffusion-limiting phenomenon. In previous reports, the adsorption of heavy metal ions to the metal oxides was said to be dominated by the process of ligand exchange with surface hydroxyl groups (-OH) on the metal oxides (Kumar et al., 2016), and tin oxide was considered to have more oxygen defects so that the hydroxyl groups are easily formed on the surface (Sun et al., 2018). The adsorption of Pb 2+ to the 20 wt% SnO x /CeO 2 is therefore very likely to occur through the surface hydroxyl groups. Focusing on the changes of Pb 2+ removal by ADS + PED, it could be seen that the difference between the ADS and ADS + PED was definitely appeared after about 10 minutes. It was therefore suggested that the photoelectrodeposition occurred via the adsorption and induced further adsorption by oxidizing the adsorbed Pb 2+ sequentially. Table 2 shows the pH changes of the reaction solution. In the ADS, the pH greatly decreased along with the progress of the adsorption of Pb 2+ . This phenomenon seems to be because H + of the surface hydroxyl groups on the 20 wt% SnO x /CeO 2 was replaced with Pb 2+ . In the ADS + PED, the pH once decreased along with the progress of the Pb 2+ adsorption. However, it could be seen that the pH increased along with the progress of the photoelectrodeposition of Pb 2+ . After the experiments of the ADS + PED, the color of the SnO x /CeO 2 suspension changed from light yellow to light brown. The adsorbed Pb 2+ was probably oxidized as shown in the following equation previously reported by Kobayashi et al., (2017).
12. Global Journal of Researches in
?????????????????????????
+ ??? ? ?? ? + ? +(3)???? 2 + + 2?? 2 ?? + 2? + ? ?????? 2 + 4?? + (4) According to the above equation, the pH should decrease. It was hence considered that the increase of the pH was due to the elution of the surface hydroxyl groups (-OH) from the 20 wt% SnO x /CeO 2 surface in the process of PbO x deposition on the surface. Fig. 6a shows the Pb 2+ removal in the various initial pH and Fig. 6b shows the point of zero charge (pH PZC ) of the 20 wt% SnO x /CeO 2 . As shown in Fig. 6a, it could be seen that the removal performance of Pb was drastically decreased at the pH below 3.5. The following three factors were assumed. (1) Since H + was released in the formation process of the surface hydroxyl groups, this process hardly proceeded under the acidic conditions. (2) In the adsorption process, Pb 2+ displaced H + of the surface hydroxyl groups. Thus, it was unfavorable under the acidic conditions. (3) The estimated pH PZC of the 20 wt% SnO x /CeO 2 was 5.3 (Fig. 6b). Thus, under the acidic conditions, the surface hydroxyl groups were positively charged (-OH 2
13. +
) and electrostatically repelled with Pb 2+ (Ferreira et al., 2015). It was therefore considered that the 20 wt% SnO x /CeO 2 photocatalysts are not suitable for use under the acidic conditions.
14. c) Mechanism of Pb 2+ removal
The mechanism of the adsorption and the photoelectrodeposition of Pb 2+ by the 20 wt% SnO x /CeO 2 photocatalysts is shown as the schematic diagram in Fig. 7. When the UV light is irradiated on the 20 wt% SnO x /CeO 2 , the electrons (e -), which are ejected from valence band (VB) of the CeO 2 , move to the conduction band (CB) by creating the holes (h + ) in the VB (Equation ( 5)). While most of the electrons are recombined with the holes and disappeared, some electrons are quickly shifted to the SnO x amorphous phase, and some holes are migrated to the CeO 2 surface (Priyadharsan et al., 2017). By the action of the generated carriers, Pb 2+ adsorbed with the surface hydroxyl groups on the SnO x is oxidized. The following three hypotheses are conceivable as a route to explain the oxidation reaction. (1) The holes on the CeO 2 surface act on nearby Pb 2+ adsorbed on the SnO x , and Pb 2+ is oxidized (Kobayashi et al., 2017). (2) The holes on the CeO 2 surface generate hydroxyl radicals (?OH) from OH -(Equation ( 6)), and they oxidize Pb 2+ on the SnO x surface (Kumar et al., 2017). (3) The electrons on the SnO x surface generate superoxide radicals (O 2 ? -) from dissolved oxygens (O 2 ) (Equation ( 7)). The superoxide radicals react with H + and produce peroxide radicals (?OOH) (Equation ( 8)). These peroxide radicals finally interact with the electrons and form additional ?OH radicals (Equations ( 9)-( 10)) (Wang et al., 2017). As the adsorbed Pb 2+ is then deposited as PbO x on the photocatalyst surface (Equation ( 11)), some adsorption sites are regenerated, and the restriction of adsorption equilibrium is resolved. As a result, removal of Pb 2+ further proceeds. The removal of Pb 2+ is finally terminated by occupying all adsorption sites with the deposited PbO x . The complete oxidation process can be understood by the following reaction steps (Kobayashi et
?????? ?? /?????? 2 + ????????????? (???) ? ?? ? (????) + ? + (????) (5) ???? ? + ? + (?????? 2 ????) ? ? ???? (??????????????)(6)?? 2 + ?? ? (?????? ?? ????) ? ?? 2 ? ? (??????????????)(7)?? 2 ? ? + ?? + ? ? ?????? (??????????????) (8) 2 ? ?????? ? ?? 2 ?? 2 + ?? 2(9)?? 2 ?? 2 + ?? ? (?????? ?? ????) ? ? ???? + ???? ?(10)???? 2+ + ? + (?????? 2 ????),? ???? + ??????. ? ?????? ?? + ??????. (11) d) Removal of other heavy metal ions
???? 2+ + 2?? (?????? ?? ????) ? ????(12)SeO 3 2and AsO 3 3were efficiently adsorbed on the surface of the 20 wt% SnO x /CeO 2 , because oxygen atoms of the oxide anions easily entered to the oxygen defects or coordinated to the Sn 4+ exposed on the surface of the amorphous SnO x (Di et al., 2006). According to the XPS analyses, it was revealed that Se 4+ and As 3+ were deposited on the surface as SeO 2 and As 2 O 3 after the ADS + PED. In contrast, Cr 2 O 7 2could not be adsorbed at all on the surface of the 20 wt% SnO x /CeO 2 . It was therefore suggested that the 20 wt% SnO x /CeO 2 cannot adsorb anything if adsorbate is an oxide anion. However, the reduction of Cr 6+ by the excited electrons was slightly occurred (Ku et al., 2001). Thus, it can be suggested that the 20 wt% SnO x /CeO 2 photocatalysts have a potential to remove various toxic heavy metal ions irrespective of cations or anions.
The 20 wt% SnO x /CeO 2 could remove heavy metal ions at low concentrations by the adsorption and sequential photoelectrodeposition processes under the UV light irradiation. However, for practical applications, further improvement of adsorption capacity is desired (Fu et al., 2011). According to the previous reports, it was indicated that the adsorption performance can be enhanced by making the photocatalyst porous or loading the photocatalyst in a highly dispersed state on the substances having a high specific surface area such as zeolite, (graphene oxide, etc Setthaya et al., 2017;Li et al., 2018;Nozaki et al., 2018).
IV.
15. Conclusion
The amorphous SnO x(1<x<2) loading on a high crystallinity CeO 2 photocatalysts were synthesized by the polymerized complex method and the impregnation method for the adsorption and photoelectrodeposition of toxic heavy metal ions. The obtained photocatalysts were characterized by XRD, XPS, SEM, BET, and pH PZC . According to the XRD and XPS analyses, the loaded SnO x was composed of Sn 2+ and Sn 4+ and possessed numerous surface hydroxyl groups. In the removal of Pb 2+ , a synergistic interaction between the photocatalytic activity of the CeO 2 and the adsorption ability of the SnO x appeared efficiently when the amounts of SnO x was 20 wt%. The photoelectrodeposition of Pb 2+ occurred via adsorption of Pb 2+ to the SnO x with surface hydroxyl groups and progressed further removal. It was therefore indicated that the photoelectrodeposition showed a promoting effect of adsorption. In the acidic pH conditions, Pb 2+ was almost not removed. Thus, it was suggested that the influence of surface hydroxyl groups of the 20 wt% SnO x /CeO 2 was dominant for the adsorption of Pb 2+ . A detailed Pb 2+ removal mechanism was devised, suggesting the oxidation of Pb 2+ by the action of holes and hydroxyl radicals. Furthermore, it was revealed that SnO x /CeO 2 photocatalysts have a potential to remove various toxic heavy metal ions irrespective of cations or anions. It was therefore shown that SnO x(1<x<2) /CeO 2 photocatalysts can purify various wastewater contaminated with heavy metal ions.
It was desired to extend the activity of the 20 wt% SnO x /CeO 2 to the removal of other toxic heavy metal ions. The removal of Cu 2+ , Cd 2+ , Hg 2+ , Se 4+ , As 3+ and Cr 6+ as well as Pb 2+ , were also investigated, and the results were shown in Fig. 8. The heavy metal ions other than Pb 2+ could also be removed by the 20 wt% SnO x /CeO 2 photocatalysts. The removal of Cu 2+ was remarkably proceeded by the photoelectrodeposition. By the XPS analysis, it was revealed that the deposited material was the metallic Cu. The reduction of the Cu 2+ adsorbed on the SnO x were efficiently proceeded because of the smooth action of the excited electrons migrated to the SnO x surface (Equation ( 12)) (Canterino et al., 2008).
| Pseudo first-order model | ||
| k 1 (/min) | q m (mg/g) | R 2 |
| 0.0152 | 0.620 | 0.941 |
| Pseudo second-order model | ||
| k 2 (g/mg/min) | q m (mg/g) | R 2 |
| 0.00338 | 0.668 | 0.966 |
| Time (min) | pH (Only ADS) | pH (ADS + PED) |
| 0 | 5.5 | 5.5 |
| 10 | 4.3 | 4.4 |
| 30 | 4.2 | 4.4 |
| 60 | 4.2 | 4.5 |
| 105 | 4.1 | 4.7 |
| 150 | 4.1 | 4.9 |