1. Introduction
he reactions of plant raw materials and food industry wastes in supercritical solvents (lower alcohols, in particular) show some promises for the full-scale production of cheap biodiesel fuel and chemicals [1][2][3][4][5][6][7][8][9][10][11][12][13][14]. First, these reactions proceed in the absence of homogeneous catalysts and therefore allow the use of a lower-grade feedstock. Second, they provide a 90-98% conversion of initial feedstock during short residence times (several tens of minutes) and thus make possible to use the reactors of flow type that considerably enhances the process efficiency. Third, these processes are free of huge amounts of waste water because there is no need to wash up the products from homogeneous alkaline or acid catalysts. Forth, all these factors enable a significant reduction in the cost of biodiesel fuel.
The use of heterogeneous catalysts in the reactions of vegetable oils with alcohols allow s [15 -to partially get rid of the homogeneous catalysts drawbacks: elimination the problem corrosion of reactor material, does not require the separation of products and acid, and makes it possible to perform the process in the continuous mode. At the same time, some poisoning of the heterogeneous catalyst is to be expected, along with a reduction in its activity and the emergence of pore diffusion resistance, resulting in a low rate of reaction. The surface of the heterogeneous catalyst used in the transesterification of triglycerides must have hydrophobic properties in order to limit glycerol and water adsorption on the active centers of the catalyst, as such adsorption results in a loss of its activity. The properties and nature of heterogeneous catalyst being used determine to a large extent the conditions for conducting the reaction and the method of products separation. Heterogeneous catalysts with acid [19,20] or basic [21,22] properties are used in triglyceride transesterification reactions.
The presence of hydroxyl group in alcohol molecule leads to the formation of glycerol at transesterification of triglycerides, which are the main constituents of all vegetable oils. The reactions of fuel synthesis from vegetable oils in methanol, including the supercritical one, yield byproduct glycerol in an equimolar amount to the amount of converted oil. Besides the need to separate glycerol from the reaction products, the problem appears how to utilize it, for example, in the synthesis of useful chemicals.
The use of the acylated alcohol instead of the lower alcohol for transesterification of triglycerides may also lead to the formation of fatty acid esters (biodiesel), but instead of glycerol another product will be formed as a byproduct. For example, the use of methyl-or ethyl acetate may lead to the formation of 1,2,3-triacetoxypropane known also as triacetin.
Triacetin is itself a valuable compound that can be used in the cosmetic and food industry or as an additive to petrol fuel [18,23]. It is also a good solvent, can be easily mixed with fatty acid esters and used as a fuel additive improving low-temperature stability and viscosity of diesel fuel (triacetin melting point is -78°C).
Methyl acetate (acetic acid methyl ester), being an acyl group donor, has been already used instead of methanol in the enzyme-catalyzed reactions of vegetable oil transesterification (see Scheme 1) [24][25][26], since methanol inhibits enzyme activity. Although enzyme-catalyzed synthesis of biofuels is proved feasible, it still has serious disadvantages restricting its wide application, such as enzyme susceptibility to the oil type and quality, large residence times to provide sufficient conversion, low process efficiency. Trying to Abstrect-The transesterification reactions of sunflower and corn oils in supercritical methyl and ethyl acetates in a tubular flow reactor without catalyst were studied. The residence time of the reaction mixture was ~2.9 min. The reaction of sunflower oil in supercritical methyl acetate yielded a large amount of free fatty acids and respective esters. The fraction of free fatty acids among the reaction products at high temperatures attained 50%. The product distributions in the transesterification reactions of vegetable oils with supercritical methyl and ethyl acetates were studied in detail. The methods of qualitative and quantitative analysis of the reaction products have been developed. perform transesterification of vegetable oils in supercritical methyl and ethyl acetates in a flow reactor.
The use of methyl acetate instead of methanol for supercritical synthesis of glycerol-free biodiesel from vegetable oils is a new process and its study is very limited in the literature. There are several studies in this field, e.g. [27][28][29][30][31][32]. The authors of [27] tested some oils with different fatty acid composition. The process was also applied to waste oil with higher free fatty acid (FFA) content. The results demonstrate that the oil composition does not significantly influence the biodiesel yield. These authors studied the influence of temperature, pressure and molar ratio of reactants by oils transesterification with supercritical methyl acetate. It has been shown that all oils achieved complete conversion after 50 min at 345 0 C and 20 MPa with methyl acetate:oil molar ratio equal to 42:1.
Kinetic of transes terification reactions of four oils with supercritical methyl acetate was studied in [27] in mixed batch reactor. Pseudo-first order equations used for modeling.
Conversion of rapeseed oil and oleic acid with supercritical methyl acetate to free acid methyl esters (FAME) and triacetin (TA) in flow tubular reactor investigated in [28] without catalyst. The results of these studies are shown that the transesterification reaction of triglycerides with methyl acetate can proceed under supercritical conditions, generating FAME and triacetin. In this work, methyl acetate (? cr = 233.7°?, ? cr = 4.63 ?P?) and ethyl acetate (? cr = 250.4°?, ?cr = 3.78 ?P?) were used in experiments for transformation of vegetables oils in continuous flow reactor.
Fatty acid methyl esters have been successfully produced from noncatalytic transesterification reaction between triglycerides (palm oil) and methyl acetate in batch-type reactor (12ml) [29]. The optimum conditions were found to be 399 0 C for reaction temperature and residense time of 59 min to achieve 97.6% biodiesel yield.
The reactions of triglycerides transesterification by methyl and ethyl acetates are assumed to proceed according to Scheme 1 and Scheme 2.
2. Experimental
Transesterification of vegetable oils with supercritical methyl and ethyl acetates was performed in stainless steel tubular flow reactor of volume ~ 23.5 cm 3 , inside diameter of reactor tube ~ 0.3 cm, its length ~ 3.3 m. The experimental setup is described in details in our earlier works [33,34]. The acetate and vegetable oil were fed to a mixer at the reactor inlet as two independent flows. The first flow was pure methyl or ethyl acetate; it was fed to the mixer by a piston pump through a heat exchanger, where it was heated to the reaction temperature. The second flow was sunflower or corn oil; it was fed directly to the mixer by a syringe pump. The "acetate/oil" parameter was calculated as a ratio of volume flow rates (cm 3 /min) of acetate and vegetable oil supplied to the reactor.
The cooled product mixture was a homogeneous non-segregating liquid, which was sampled for analysis under fixed stationary experimental conditions.
The present studies were performed using refined edible sunflower and corn oils and an ACROS ORGANICS methyl acetate (>99.8 wt.%), ethyl acetate (>99.5 wt.%).
The residence time of the reaction mixture was calculated as a ratio of the inlet acetate-oil mixture flow rate Q (cm 3 /min) to the reactor volume (23.5 cm 3 ). For example, if Q = 8 cm 3 /min, the residence time was ~2.9 min. The reaction was performed in the temperature range of 213-400°C at pressure ~ 200-220 atm. The temperature and pressure providing supercritical state of the reaction mixture were selected on the base of thermodynamic calculations and phase diagram plotting
O O OC(O)R 2 OC(O)R1 OC(O)R3 + 3 OC(O)CH 3 OC(O)CH 3 OC(O)CH 3 + R1C(O)OCH3 R 2 C(O)OCH 3 R3C(O)OCH3 1 2 3 4 O O OC(O)R 2 OC(O)R 1 OC(O)R 2 + 3 OC(O)CH 3 OC(O)CH 3 OC(O)CH 3 + R 1 C(O)OC 2 H 5 R 2 C(O)OC 2 H 5 R 3 C(O)OC 2 H 53. a) Methods of analysis
Transformations of vegetable oils with acetates are performed usually at low temperatures and in the presence of catalysts, mainly, enzymes. Under these conditions, triacetin and fatty acid methyl or ethyl esters are the main reaction products. Increased temperature and pressure will most likely facilitate the formation of other products. For this reason, in this work we focused special attention on the methods of qualitative and quantitative analysis of the reaction products.
Liquid products were analyzed by a chromatomass-spectrometer Agilent Technologies 7000 GC/MS Triple Quad, GC System 7890A, using a Zb-Wax column of length 30 m, i.d. 0.25 mm, film thickness 0.25 ?m; measurement range m/z: 40 -500. Heating protocol: 2 min at 50°C, 8°C/min to 260°C, 30 min at 260°C. Carrier gas He, ion source temperature 230°C, flow split ratio 1:20, vaporizer temperature 300°C. The products were identified by comparing the retention times and massspectra with the reference libraries NIST and Wiley7. Quantitative analysis of the fatty acids and respective esters was performed using an internal standard (1hexanol) calibration method.
The content of free fatty acids in the initial sunflower oil was determined by two methodschromate-mass-spectrometry and titration. According to chromate-mass-spectrometric data, the initial oil contained free fatty acids in the amount of ~2.75 vol.%. Note that the quantitative analysis of fatty acids was complicated by their partial adsorption on the analyzer surfaces. To improve measurement validity, after each analysis a certain amount of pure solvent was injected into the analyzer for washing out the residual fatty acids. Then the amounts of the fatty acid detected in the washout and in the sample were summated that provided correct data on the fatty acid content in the initial oil.
Titration of heated oil-isopropanol mixture with added indicator (phenolphthalein) was performed by a solution of 0.1 g NaOH in 100 g of water (0.1% NaOH aqueous solution) till the mixture turned pale pink. Then the titration was stopped and the consumed amount of the titrant was measured. According to titration analysis, initial oil contained ~2.7% of free fatty acids.
Based on the results of chromate-massspectrometric and titration analyses, the total content of fatty acids in the sunflower oil was assumed to be ~ 2.7 vol.%.
Among the indentified acids, linoleic acid showed the highest content.
4. III.
5. Results and Discussions
Before starting experiments on the oils transesterification in supercritical methyl and ethyl acetates, thermal stability of the latter has been studied. To study thermal stability of methyl and ethyl acetates, we used the above setup without oil feeding to the reactor. Thus, methyl acetate showed sufficient thermal stability in the flow reactor at temperatures 200-340°C, pressure 200 atm and residence time ~2.9 min. At temperatures above 350°C, weak gas emission was observed at the reactor outlet, and acetic acid was detected in the liquid-phase products.
It was found that supercritical ethyl acetate in a flow reactor at temperatures 250-340°C, pressure 200 atm and residence time ~4.7 min remained stable. As the temperatures exceeded 340°C, the release of gaseous products was observed; the liquid phase contained also the acetic acid. The gaseous products included hydrogen, CO, methane, CO 2 , ethane and ethylene. With the temperature increase from 360 to 450°C, the outlet concentration of acetic acid increased by more than 6 times. The yield of gaseous products increased similarly. No ethanol traces were found in the liquid phase.
Since the initial reaction mixtures contained no water, and no methanol and ethanol was detected in the reaction products, it seems hardly possible that the acetic acid appeared by the reaction of methyl or ethyl acetate hydrolysis (a reverse reactions to the synthesis of methyl acetate from acetic acid and methanol, and ethyl acetate from acetic acid and ethanol). It should be noted that methanol or ethanol formation cannot be excluded entirely -they may be formed and then rapidly consumed in the reaction of triglycerides transesterification. Thus, transformation of methyl or ethyl acetates under the reaction conditions proceeds by thermal decomposition to produce acetic acid and gaseous products. Since acetic acid appears in the reaction mixture in trace amounts, it catalytic effect on the oil transesterification reaction is insignificant.
6. a) Sunflower transesterification in supercritical methyl acetate
As shown in our earlier studies of vegetable oil (including sunflower one) transesterification with supercritical methanol [33,34], fatty acid esters and glycerol were the main reaction products.
Table 1 presents the product distribution in the reactions of sunflower transesterification in supercritical methyl acetate at various temperatures. Obviously, the qualitative and quantitative product compositions in this reaction are strongly different from those in the reaction of sunflower oil transesterification in supercritical methanol [33][34][35] under similar reaction conditions.
As the temperature increased from 380 to 400°C, the yield of glyceryl linolate and glyceryl oleate increased approximately threefold. Previously [33][34][35], we found a weak effect of pressure on the of oils transesterification reaction in supercritical alcohols. We explain this effect in the absence of significant changes in the solvent properties under pressure changes. For this reason, the effect of the pressure on the reaction rate of oils with acetates has not been studied. 1, the product content (besides triglycerides) is presented as a volume fraction of the product with regard to total volume of the analyzed compounds. The content of non-converted triglycerides is given as a volume fraction with respect to the fixed volume (V = 0.5 ?m) of the sample injected into the chromato-mass-spectrometer. The volume of nonconverted triglycerides V prod. trigl was calculated on the base of total volume balance of the reaction products:
(1) where V MA -volume of methyl acetate, V hex -volume of 1hexanol, V acet.acid -volume of acetic acid, V FAME -volume of fatty acid methyl esters, V FFA -volume of free fatty acids All components of equation ( 1) were calculated on the base of chromato-mass-spectrometric data. The results obtained showed that the content of fatty acids and respective esters in the reaction products increased with increasing reaction temperature. The most significant increase was observed for oleic and linoleic acids, and respective esters.
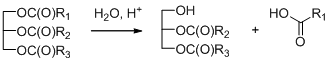
Formation of fatty acids and their increasing content in the reaction products with the increasing temperature would be explained by hydrolysis of triglycerides according to Scheme 3, if the reaction mixture contained large amounts of incompletely transesterificated triglycerides and a source of protons.
7. Scheme 3
However, in our experiments incompletely transesterificated triglycerides, such as glyceryl linolate and glyceryl oleate, appeared in small and almost equal amounts only at high temperatures. Moreover, although the content of all fatty acid esters significantly increased with increasing temperature, small amount of triacetin was detected only at 347°C; at higher temperatures it increased almost twice.
The fractions of methyl oleate and methyl linolate increased most strongly with increasing temperature (Table 1).
The oil conversion was calculated according to equation: Conversion = (1? prod trigl ) ? 100%, where ? prod trigl -volume fraction of triglycerides in the reaction products, calculated by eq. ( 2): Year 2016 C V prod. trigl. = V -V MA -V hex . -V FAME -V FFA -V acet.acid , (2) where V 0 trigl -volume of triglycerides in the initial oil; V prod trigl -volume of triglycerides in the reaction products, calculated by eq. ( 1).
Precise determination of the free fatty acid content in the initial oil makes possible to calculate the content of triglycerides (V 0 trigl ).
As the residence time was increased from 2.9 to 5.9 min, the oil conversion and the yield of the target products (methyl esters of fatty acids) increased considerably (Table 2). It is clearly seen that the temperature increase of 20°C at this residence time caused a three-fold increase in the esters yield. This result seems very important, because slight elongation of the tubular reactor makes feasible to reach complete oil conversion at a lower reaction temperature. 3 presents the product distribution (after deduction of acetic acid and ethyl acetate) for the reaction of sunflower oil transesterification by supercritical ethyl acetate at various temperatures. Note, the qualitative and quantitative product compositions in this reaction vary strongly from those in the reaction with supercritical methanol [33,34], other conditions being the same. The main differences are low content of fatty acid esters, high content of free fatty acids and incompletely substituted products. It is seen that even at low temperatures the reaction products contain fatty acids that proves the presence of initial oil. Analysis of initial oil supports this suggestion. However, the content of fatty acids increases slightly with increasing temperature, i.e. they form during the reaction. It is reasonable to suggest that the free fatty acids are formed during acid-catalyzed hydrolysis of triglycerides.
8. ii. Reaction of corn oil transesterification with ethyl acetate
The studies showed no significant differences in the reactions of sunflower and corn oils transesterification in sc ethyl acetate (Table 4). In both cases, formation of free oleic and linoleic acids was observed with increasing temperature; no traces of triacetin-the product of complete grycerin transesterification -were detected. IV.
9. Global
10. Conclusions
Although transesterification of vegetable oils with supercritical methyl and ethyl acetates has some advantages over this process with lower alcohols (methanol, ethanol), the obtained products are still below the biofuel quality standards at the selected parameters of reaction. The reaction of oils with supercritical methyl acetate yields fatty acid esters and free fatty acids. The fraction of the latter in the reaction products attains up to 50% at high temperatures.
Product distribution at transesterification of sunflower and corn oils in supercritical ethyl acetate was quite different -it showed small content of fatty acid esters, and high content of free fatty acids and partially (incompletely) substituted triglycerides.
Nevertheless, the obtained data on vegetable oils conversion in supercritical methyl and ethyl acetates at the same fixed parameters in a flow reactor at short residence times are the starting point for the optimization of the transformation conditions providing the desired product composition.
11. Global

| [35]. |
| Year 2016 |
| 22 |
| Global Journal of Researches in Engineering ( ) Volume XVI Issue I Version I C |
| Sample | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| ?, ? ? | 27 | 213 | 271 | 301 | 334 | 347 | 380 | 400 |
| Methyl acetate | 0.967 | 0.924 | 0.924 | 0.904 | 0.838 | 0.743 | 0.556 | 0.426 |
| 1-Hexanol | 0.017 | 0.022 | 0.018 | 0.016 | 0.018 | 0.020 | 0.011 | 0.010 |
| Acetic acid | 0.0008 | 0.0014 | 0.0006 | 0.0007 | 0.002 | 0.003 | ||
| Methyl palmitate | 0.0003 | 0.0005 | 0.003 | 0.005 | ||||
| M w =318 | 0.0002 | 0.0004 | 0.004 | 0.007 | ||||
| Methyl stearate | 0.0002 | 0.0005 | 0.002 | 0.005 | ||||
| Methyl oleate | 0.0004 | 0.0010 | 0.006 | 0.011 | ||||
| Methyl linoleate | 0.001 | 0.003 | 0.023 | 0.042 | 0.172 | 0.262 | ||
| Sum of esters | 0.001 | 0.003 | 0.0241 | 0.0444 | 0.186 | 0.290 | ||
| Triacetin | Traces | Higher than 4 | Higher than 5 | Higher than 6 | ||||
| Palmitic acid | 0.005 | 0.008 | 0.009 | 0.015 | 0.021 | 0.036 | 0.042 | 0.047 |
| Stearic acid | 0.004 | 0.003 | 0.007 | 0.015 | 0.015 | 0.018 | ||
| Oleic acid | 0.003 | 0.011 | 0.008 | 0.013 | 0.019 | 0.030 | 0.048 | 0.049 |
| Linoleic acid | 0.007 | 0.036 | 0.035 | 0.044 | 0.073 | 0.112 | 0.140 | 0.157 |
| Sum of acids | 0.015 | 0.054 | 0.056 | 0.075 | 0.119 | 0.192 | 0.245 | 0.271 |
| Triglycerides | 0.361 | 0.360 | 0.365 | 0.359 | 0.308 | 0.297 | 0.178 | -0.048** |
| Conversion, % | 1.89 | 2.11 | 0.68 | 2.30 | 16.13 | 19.16 | 51.61 | ~100.0 |
| Sample | 1 | 2 |
| ?, 0 ? | 330 | 350 |
| Volume fraction *) | ||
| Palmitic acid methyl ester | 0.08 | 0.18 |
| Stearic acid methyl ester | 0.03 | 0.08 |
| Oleic acid methyl ester | 0.07 | 0.17 |
| Linoleic acid methyl ester | 6.96 | 20.25 |
| *) Volume fraction was calculated as a ratio of | ||
| the ester volume to the fixed volume (5 ?m) of the | ||
| sample injected to the analyzer | ||
| b) Transesterification of sunflower and corn oils by ethyl | ||
| acetate | ||
| i. Reaction of sunflower oil with ethyl acetate | ||
| Table |
| Temperature , ? ? Product ,vol. % | 260 | 300 | 340 | 360 | 400 | 425 |
| glyceryl 1,2-diacetate | 0.61 | 1.03 | 5.02 | |||
| palmitic acid ethyl ester | 1.48 | 3.88 | 6.35 | |||
| stearic acid ethyl ester | 0.95 | 2.64 | 3.04 | |||
| oleic acid ethyl ester | 0.89 | 2.8 | 11.14 | 12.22 | ||
| linoleic acid ethyl ester | 1.96 | 4.2 | 13.83 | 11.18 | ||
| Sum of esters | 2.85 | 9.43 | 31.49 | 32.79 | ||
| palmitic acid | 2.7 | 2.6 | 2.64 | 2.77 | 3.85 | 3.3 |
| stearic acid | 1.1 | 1.1 | 1.08 | 1.57 | 2.22 | 2.3 |
| oleic acid | 6.0 | 6.6 | 6.85 | 7.83 | 10.86 | 9.71 |
| linoleic acid | 7.8 | 13.1 | 15.06 | 13.63 | 12.75 | 11.55 |
| Sum of acids | 17.6 | 23.4 | 25.63 | 25.8 | 29.68 | 26.86 |
| glyceryl palmitate, 2,3-diacetate | 2.14 | 0.98 | 4.3 | |||
| glyceryl oleate, 2,3-diacetate | 1.59 | 3.45 | 2.92 | |||
| glyceryl linoleate | 0.67 | 8.66 | 4.39 |
| Year 2016 |
| 25 |
| C |