1. I. Introduction
yrolysis remains an important process for thermo-chemical coal conversion either as an individual process for char and tar/oil production or as a step in other coal conversion processes such as combustion, gasification, and liquefaction. During fixed bed gasification, coal passes through four distinct stages: drying, pyrolysis, reduction, and combustion [1]. In the pyrolysis stage, volatiles devolatilization occurs and the properties transformation from coal to char can be dramatic for some coals. The behavior of coal under pyrolysis can be linked to coal properties and process conditions such as coal rank, particle size, porosity, surface area, mineral content, petrographic composition, process temperature, process pressure, catalyst, and heating rate [2 -13]. Thus, among other poor and unstable performance of coal conversion processes [8, 14 -17]. However, in other conversions, such as direct liquefaction the pyrite mineral is beneficial to the yield of the process [3,12].
The atomic H/C, aromaticity, petrography, and fuel ratio (the ratio of fixed carbon to volatile matter content) influences on coal conversion processes have been investigated [18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33]. Char properties such atomic H/C ratio, aromaticity, morphology and fuel ratio has also received attention [8, 19, 25, 34 -36]. However, a systematic evaluation for the transitions accompanying slow-heating rate using a temperature monitored process is limited [37][38][39][40]. Zhao et al. [37] examined the pyrolysis behaviour of vitrinite and inertinite extracts from a Chinese bituminous coal at temperatures of 400 to 650 o C and heating rate of 10 o C/min and reported that the atomic H/C ratio of both the vitrinite and inertinite char decreased with increasing temperature, and have similar atomic H/C ratio and structure characteristics at the final temperature. Guerrero et al. [38] performed combined reflectance analysis on heattreated coal at temperatures of 400, 450, 550, 750, 1000 o C and reported the effect of volatiles release on the optical properties of macerals from different ranks The reflectance of coal macerals increases with increasing pyrolysis temperature, with temperature being the most relevant factor in determining the reflectance of inertinite macerals. The studies of Jimenez et al. [39] and Alonso et al. [40] both focused on the influence of process conditions on char optical texture and reactivity. They reported the influence of both pressure and temperature on the resultant chars. The coal char properties derived at 900 o C and heating rate of 20 o C/min, exhibited significant changes in reactivity in the pressure range 0.1 to 0.5 MPa, after which no significant changes were observed with pressure increment. Alonso et al. observed that the temperature of pyrolysis performed at temperatures of 1000 and 1300 o C had a strong effect on char reactivity, which was attributed to inertinite maceral in high rank coals yielding both more reactive and less reactive materials depending on the process conditions. Tracing char structure evolution during coal conversion indicators and char properties the fuel ratio (a commonly used factor in the design of unit operations for coal conversion processes and also a useful parameter in the selection of coals used in coal blending plant) has not commonly evaluated but may prove to be useful indicator in the coal to char conversion process [3,7]. Most power plants utilized coals with fuel ratio in the range of 1.0 -2.5 due to their combustion characteristics of calorific values, ignitability, and combustibility [4,7,33]. Fuel ratio is considered to give an indication of coal reactivity as coals with fuel ? 1.5 are thought to combust more smoothly whereas coal with fuel ratio ? 1.5 combust with difficulty [2,4,31]. Kurose et al. [4] examined the pulverized coal combustion characteristics of high-fuelratio coals with fuel ratio in the range of 1.46 to 7.10 and concluded that as the fuel ratio increases, nitrogen oxides (NO x ) emission reduces significantly and that the char's external surface area decreases with increase in the fuel ratio of the coal. Fuel ratio of acid washed and slow-heating coals are not readily available. Thus the fuel ratio is a somewhat forgotten rank parameter that may have utility in coal to char transitions.
Here the chemical and structural changes accompanying coal pyrolysis performed on a 75 ?m size, at a heating rate of 20 o C/min from 450 to 700 o C at atmospheric pressure. The changes in the char structure were traced and relationship between char formation process indices and coal/char properties are established. The selected coals were acid washed to reduce mineral matter and ash influences.
2. II. Experimental a) Sample Preparation
Six coals of varying rank were used: a lignite coal from Germany (LIG); a sub-bituminous coal from Nigeria (SUB); two South African bituminous coals (one is low volatile bituminous (BIT-LV) and the other, high volatile bituminous coal (BIT-HV); South African semianthracite (SA); and anthracite from South Africa (ANT). The coal samples were pulverized to coal particle size of ? 75 ?m by employing a mechanical size reduction jaw crusher (Samuel Osborne (SA) LTD, model: 66YROLL) and a Fritsch P-14 rotary mill containing ceramic balls (Model number: 46 -126). The required particle size of -75 µm was finally obtained from screening the particles from the rotary mill using a 75 µm screen All the samples were stored under argon in sealed bags.
The prepared coal samples were acid washed by sequential leaching with hydrofluoric acid (HF) and hydrochloric acid (HCl) as detailed in Strydom et al. [17].The HF (48%) and HCl (32%) were obtained from Associated Chemical Enterprise (ACE), South Africa.
3. b) Apparatus and procedure
The coal samples (40g) were placed in a ceramic boat in a horizontal tube furnace at atmospheric conditions initially. The samples were flushed with nitrogen (AFROX, ultra high purity grade) at atmospheric conditions, to remove oxygen from the oven for 15 min. at a flow rate of 1 litre/min. The furnace was then heated at 20 o C/min to the target temperature, and held isothermally for 60 minutes. The target temperature ranged from 450 to 700 o C, while keeping the samples under a nitrogen atmosphere. The char samples were stored in sealed bags.
The calorific value and conventional chemical analyses (proximate and ultimate analyses) of the untreated coal, acid treated and heat treated samples were performed according to the ISO 1928, ASTM 3172 and ASTM 3176 standards respectively at Advanced Coal Technology (ACT), Pretoria, South Africa. The surface areas of the various samples were determined using the carbon dioxide adsorption BET method on a Micromeritics ASAP2020 surface area analyser [41]. Prior to CO 2 adsorption, the samples (about 0.20 g) were degassed under vacuum (10.0 µmHg), for 48 hours at 25 and 380 o C for the coals and chars respectively. The evacuated sample was analysed at 0 o C in an ice bath. The results were processed using the Accelerated Surface Area and Porosimetry System (ASAP) 2020 software linked to the Surface Area Analyzer. The spectra used in obtaining the structural properties of both the coal and char were obtained from the Fourier-transform infrared spectrometer equipped with an attenuated total reflectance (FTIR-ATR), model Perkin-Elmer Spectrum 400. The procedure of FTIR-ATR as detailed by Li et al. was used [42]. Aromaticity (f a ) was obtained from the ratio of aromatic bands in the 900 -700 cm -1 region to the aliphatic and aromatic bands in the 3000 -2815 cm -1 region [43]. The vitrinite reflectance of the parent coal was obtained following the procedure and equipment at the coal and carbon laboratory, University of the Witwatersrand, South Africa as detailed in Malumbazo et al. [29].
4. III. Results and Discussion
Chemical analysis for the parent coal are summarized in Table 1, where coals are listed by increasing rank (lignite to anthracite) as determined by vitrinite reflectance analysis [29]. The rank parameters follow the expected trend with increasing rank, a decrease in volatile matter, hydrogen content, oxygen content, atomic O/C ratio and atomic H/C ratio, and an increase in carbon content, fuel ratio, and aromaticity [8 -9, 12 -15, 18 -22, 54 -55]. The atomic ratio of hydrogen to carbon (H/C) increases as the coal rank decreases with lignite coal sample having the highest ratio of 1.12 and the anthracite coal has the lowest value of 0.36. A similar trend was obtained for the atomic O/C ratio with the lignite coal having a value of 0.20 and the anthracite coal, 0.02. The values obtained in this study compare well with those of Ibarra et al. [18], who reported values ranging from 1.27 for peat to 0.17 for anthracite for the atomic H/C ratio and 0.43 for peat and 0.06 for anthracite. The aromaticity shows a trend of increasing values with increasing coal rank with the lignite coal having a value of 0.38 and the anthracite a value of 0.97 [17 -18, 25]. A similar trend was obtained for the calorific value with the lignite having a value of 21.2 MJ/kg and the anthracite a value of 29.6 MJ/kg. Typically there is a slight drop in the calorific value between the low rank and high rank coals. The fuel ratio ranges from 0.6 for the lignite coal to 16 for the anthracite coal. There was good agreement with the aromaticity, calorific value, and fuel ratio of the bituminous coals (BIT-LV and BIT-HV) with those reported by Everson et al. for typical South African bituminous coals [25].
The lignite coal has a mean random vitrinite reflectance of 0.35 representing a low rank C coal (Table 1). The mean random vitrinite reflectance obtained for the sub-bituminous coal in this study was 0.47 characterised as low rank B coal; for the bituminous coals, the values determined are 0.73 and 0.78 for BIT-LV and BIT-HV characterised as medium rank C coals. The high rank coals SA and ANT have values of 2.48 and 3.26 thus high rank C and high rank B coals respectively [28 -29].
The chemical analyses of the acid-washed coal are presented in Table 2. The differences observed between parent and acid washed coal was significant. A similar trend was observed for the acid washed coals as was with the parent coals. Observations of increased surface area with coal rank for acid cleaned were also reported by Kister et al. [12] The increased volatile matter as a result acid washing was expected to have increased the surface area and porosity of the coals due to the opening of pores, which were blocked by mineral impurities, but that was not the case with lower rank coals that experienced higher percentage increase in volatile matter content [12]. However, that was not observed for the low-rank coals where the surface area was reduced or unchanged. Mahajan and Walker [16] also observed a reduction in surface area for some coals, attributed to mineral precipitation or blocking of pores. This finding of insignificant change in aromaticity was reported by the work of Strydom et al. [17], who reported no change in the aromaticity of the demineralized coals when compared to the raw bituminous coals, and by the work of Ishihara et al. [33], who investigated the effect of demineralization on hydrogen transfer of coal with tritiated gaseous hydrogen. They revealed that demineralization does not affect the amounts of functional groups in the parent coals of different ranks ranged from lignite to low volatile bituminous coal. Here, the atomic H/C was not significantly changed and the O/C was relatively constant after the acid wash. This is similar to the observations of Strydom et al. [17] and Kister et al. [12].
Table 3 shows the atomic H/C ratio decreases with increasing char formation temperature. At temperature of 700 o C, there was a convergence to the value of 0.13 which was also observed by Zhao et al. [37]. As can be seen in Table 3, the O/C atomic ratio decreases with increasing char formation temperature for all the coals apart from anthracite with constant value of 0.03 for the temperature range of 450 -700 o C. The uniform value obtained for the anthracite can be explained by variation in petrographic composition (data not included in this paper). The anthracite may be considered as a typical South African high-inertinite content coal. The aromaticities show an increasing trend with increasing char formation temperature (Table 3). For instance, Everson et al. [25] had similar aromaticities and trends for raw and acid-washed chars heat treated in a similar range (550 to 850 o C).
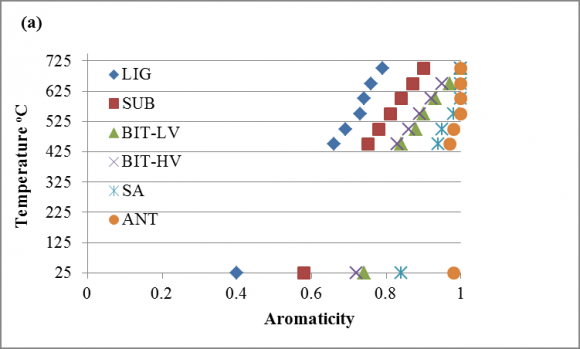
The fuel ratio data is given in Table 3. The fuel ratio increases with increase in the char formation temperature process. Though slight differences can be observed in the values obtained for the parent coal and the acid washed coals, the impart is more significant in the heat treated coal and a convergence occur around 700 o C especially for the low and medium ranks (Table 3). During the char formation process in the temperature 450 -700 o C, devolatilization, aromatization, and arrangement of the basic structural units (BSUs) are likely to have occurred as there were significant transformational changes in aromaticity, fuel ratio, H/C and O/C obtained. The pattern of these transformations in aromaticity, fuel ratio and H/C was consistent for all coal chars: lignite to anthracite. The H/C values decreases with increase in the heating temperature while the aromaticity and fuel ratio increases with increase in heating temperature as seen in Figure 1. This is attributed to the chemical modification (devolatilization, removal of aliphatic groups and heteroatoms) that occurs during carbonization that results in the ordering of the internal structure of the samples [25, 43 -45]. From these parameters it can be observed that coal increases its aromaticity and fuel ratio with increasing char formation temperature. Aromaticity and atomic H/C ratio are in use as reference indicators in coal conversion processes. It is suggested that fuel ratio has similar utility.
To establish a relationship between the determined aromaticity, fuel ratio and H/C ratio during the char formation temperatures of 450 -700 o C; figures of the transitional relationships with Global Journal of Researches in Engineering ( ) Volume XV Issue IV Version I temperature, aromaticity with atomic H/C, fuel ratio versus aromaticity, fuel ratio versus H/C were constructed (Fig. 1 -7). In Fig. 1, the changes in fuel ratio, aromaticity and atomic H/C of the parent coals are compared with the chars obtained at increasing char formation temperature. The fractional increase in the fuel ratio was more pronounced in the low and medium ranks with increasing char formation temperature as expected. The same trend was observed for the aromaticity, whereas, for the atomic H/C, fractional decrease was observed. Fig. 2 and 3 reveals the relationship between the aromaticity and atomic H/C ratio. The correlation between the aromaticity and H/C ratio gave a linear relationship (Fig. 2), whereas for the coal char, the relationship obtained was dependent on the coal rank (Fig. 3). These results are consistent with the general trends reported in previous studies for raw bituminous coal but the extension of these correlations to interpret the structural transformation of other coal rank remain a subject of controversy [46 -49]. Whereas the correlation obtained in this study is dependent on coal rank and could be used for the interpretation of the relationship between aromaticity and atomic H/C structural changes (Fig. 3). Fig. 4 demonstrates the correlation between aromaticity and fuel ratio with increasing char formation temperature, which follows same trend for all coal ranks apart from the low rank coals with little degree of deviation from the trend. This deviation could be attributed to the higher percentage of oxygen and oxygen containing functional groups in the low rank coals (LIG and SUB). It is known the low-rank coals have limited thermoplastic ability and hence do not undergo as extensive an ordering as bituminous coals The two low-rank coals do not align and coalesce to the same degree resulting in a lower aromaticity. These results of increasing aromaticity with increasing char formation temperature are consistent with the general trend reported in previous charring studies on raw coal in respect of increasing aromaticity with decreasing coal reactivity [50]; increasing aromatic condensation [51]; less ordered material [52]; increasing reflectance [32]; and increasing optical texture [38]. Fig. 5 presents the variation of fuel ratio with atomic H/C. With increasing fuel ratio with the char formation temperature, there is a corresponding decrease in the atomic H/C with a convergence to the same value of 0.13 at the final char formation temperature of 700 o C. The fuel ratio for the two low rank coals were close with similar devolatilization behavior, the two bituminous coals with close fuel ratio values have a similar devolatilization behavior, while the two high rank coals behave in like manners when subjected to heat treatment.
Table 3 also shows the surface area decreasing with increasing rank in the char formation process. Figs. 6 indicates the variation of the BET surface area with the atomic O/C (lignite coal is used as an example for illustration) and % change in BET surface area with % change in oxygen (daf). The variation in atomic O/C and % change in oxygen and compared to the raw coal is more pronounced in the low rank coals, due to higher oxygen content and of oxygen-containing functional groups such as carboxylic and phenolic [19,[53][54]. Coal particles undergo thermo-chemical decomposition with release of volatile will often increase in the porosity and surface area of the resultant char [52,[55][56][57]. The relationship in Fig. 6 showed that for every decrease in the atomic O/C, there is a corresponding increase in the surface area during the char formation process. It is generally known that the diffusion of oxygen to and within the char particle influences the rate of char burning and resulting transitions in char morphology (Fig. 5.) [19,40,[58][59].
5. Conclusion
Though previous research have reported the characterization of coal and the subsequent chars, analysis on the transition stage with emphasis on the organic component only have not been adequately investigated. This study entailed the characterization of conventional and FTIR technique. It can be concluded that all different rank of coals show a similar behaviour in char properties, when subjected to elevated temperatures. The volatile matter, hydrogen, oxygen, atomic O/C ratio and atomic H/C ratio, decrease with Global Journal of Researches in Engineering value, fuel ratio and aromaticity increase with charring temperature, with the low rank coals (LIG and SUB) showing larger fractional structural changes. The crystallite structure order here as measured by aromaticity for the acid washed coal chars increases slightly with increase in heat treatment temperature for the higher rank coals. Regardless of the rank, all the acid washed coal chars become increasingly similar in atomic H/C at 700 o C. The same trend was revealed in the aromaticity for the medium to high rank coals at temperature of 700 o C. The data generated for the fuel ratio (1.9 -21.0 for LIG; 2.9 -20.3 for SUB; 5.5 -24.0 for BIT-LV; 6.0 -24.1 for BIT-HV; 11.6 -29.6 for SA and 16.5 -27.8 for ANT, respectively), may be considered as a useful resource in coal processing data base. Therefore, I suggest the fuel ratio as a somewhat forgotten rank parameter that has utility in char formation work to be reconsidered for this purpose. Finally, it was possible to find a linear relationship between the coal char aromaticity and atomic H/C.
V.





| Year 2015 | |||||||
| 8 | |||||||
| IV Version I | |||||||
| e XV Issue | |||||||
| ( ) Volum E | |||||||
| Global Journal of Researches in Engineering | Coal Inherent moisture(air dried) wt.% Ash (air-dried) wt.% Volatile matter (air-dried) wt.% Fixed carbon (air-dried) wt.% Carbon (daf) wt.% Hydrogen (daf) wt.% | LIG 15.4 12.4 45.7 26.4 70.5 6.6 | SUB 9.6 9.0 37.6 43.8 75.6 5.2 | BIT-LV 4.2 29.1 21.4 45.3 77.5 4.5 | BIT-HV 2.1 16.2 26.7 55.0 81.6 4.6 | SA 1.0 17.3 7.6 74.1 90.4 3.5 | ANT 1.5 11.2 5.3 82.0 90.2 2.7 |
| Nitrogen (daf) wt.% | 0.6 | 1.7 | 2.2 | 2.0 | 2.0 | 2.2 | |
| Oxygen (daf) wt.% | 18.5 | 16.9 | 15.4 | 10.7 | 3.3 | 2.7 | |
| Sulphur (daf) wt.% | 3.7 | 0.7 | 0.4 | 1.2 | 0.9 | 2.3 | |
| Gross calorific value (MJ/kg) (air-dried) | 21.2 | 24.6 | 20.0 | 26.8 | 28.7 | 29.6 | |
| H/C (daf). | 1.12 | 0.82 | 0.69 | 0.67 | 0.46 | 0.36 | |
| O/C (daf) | 0.20 | 0.17 | 0.15 | 0.10 | 0.03 | 0.02 | |
| f a | 0.38 | 0.57 | 0.70 | 0.71 | 0.91 | 0.97 | |
| Fuel ratio | 0.6 | 1.2 | 2.1 | 2.1 | 10 | 16 | |
| CO 2 BET surface area (m 2 /g) | 74 | 104 | 95 | 84 | 108 | 122 | |
| Rank (mean random vitrinite reflectance %) | 0.31 | 0.47 | 0.78 | 0.73 | 2.48 | 3.26 | |
| © 2015 Global Journals Inc. (US) |
| Coal | LIG | SUB | BIT-LV | BIT-HV | SA | ANT |
| Inherent moisture(air dried) wt.% | 1.7 | 1.9 | 1.3 | 2.7 | 2.3 | 2.5 |
| Ash (air-dried) wt.% | 0.8 | 2.0 | 3.3 | 1.2 | 1.8 | 1.5 |
| Volatile matter (air-dried) wt.% | 60.3 | 43.2 | 25.0 | 27.2 | 9.6 | 6.8 |
| Fixed carbon (air-dried) wt.% | 37.3 | 53.0 | 70.4 | 68.9 | 86.3 | 89.2 |
| Carbon (daf) wt.% | 69.2 | 75.1 | 80.9 | 83.4 | 89.0 | 85.6 |
| Hydrogen (daf) wt.% | 6.2 | 5.2 | 4.2 | 4.6 | 3.3 | 2.4 |
| Nitrogen (daf) wt.% | 0.6 | 1.8 | 2.3 | 2.0 | 1.8 | 2.0 |
| Oxygen (daf) wt.% | 20.3 | 17.4 | 12.3 | 9.1 | 5.0 | 7.7 |
| Sulphur (daf) wt.% | 2.7 | 0.1 | 0.3 | 1.0 | 0.7 | 2.1 |
| Gross calorific value (MJ/kg) (air-dried) | 28.9 | 29.3 | 30.0 | 32.0 | 33.3 | 32.7 |
| H/C (daf) | 1.08 | 0.83 | 0.62 | 0.66 | 0.45 | 0.34 |
| O/C (daf) | 0.22 | 0.17 | 0.11 | 0.08 | 0.04 | 0.07 |
| fa | 0.40 | 0.58 | 0.74 | 0.72 | 0.84 | 0.98 |
| Fuel ratio | 0.6 | 1.2 | 2.8 | 2.5 | 9.0 | 13.0 |
| CO2 BET surface area (m2/g) | 74 | 94 | 111 | 95 | 125 | 138 |
| Year 2015 | |||||
| 9 | |||||
| CHAR LIG 450 500 | Wt % (air dried) Mois. Ash V.M F.C 2.8 1.5 32.6 63.0 82.9 3.1 1.0 10.4 2.5 0.10 0.45 0.66 1.9 Wt % (daf) daf daf C H N O S O/C H/C f a FR 2.5 1.7 29.8 66.0 85.1 2.8 1.0 8.4 2.6 0.08 0.40 0.69 2.2 | m 2 /g BET 170 194 | XV Issue IV Version I | ||
| 550 600 650 700 SUB 450 500 550 600 650 700 BIT-LV 450 500 550 600 650 700 BIT-HV 450 500 550 | 2.4 2.1 2.5 3.1 3.2 3.1 3.0 3.2 3.6 4.0 1.5 1.3 1.0 0.8 0.8 1.0 0.9 1.1 0.9 | 1.7 22.3 73.6 88.9 2.1 1.0 5.8 2.3 0.05 0.28 0.73 2.3 1.8 9.8 86.2 90.4 2.1 1.1 4.1 2.3 0.04 0.28 0.74 8.8 1.8 6.7 89.0 91.8 1.6 1.1 3.3 2.3 0.03 0.21 0.76 13.3 2.4 4.3 90.1 93.1 1.0 1.0 2.6 2.3 0.01 0.13 0.79 21.0 2.7 24.2 69.9 84.9 3.2 2.2 9.2 0.5 0.08 0.45 0.75 2.9 2.3 22.5 72.1 87.3 2.7 2.3 7.2 0.5 0.06 0.38 0.78 3.2 1.9 21.7 73.3 89.7 2.4 2.3 5.1 0.5 0.05 0.32 0.81 3.4 2.4 13.3 81.1 91.3 2.0 2.3 4.1 0.5 0.04 0.26 0.84 6.1 2.5 8.8 85.1 92.0 1.5 2.2 3.9 0.5 0.03 0.16 0.87 9.7 2.5 4.4 89.1 93.1 1.0 2.0 3.4 0.5 0.03 0.13 0.90 20.3 3.6 14.6 80.3 88.1 3.1 2.1 6.4 0.4 0.06 0.42 0.84 5.5 3.7 12.5 82.5 89.7 2.7 2.2 5.1 0.3 0.05 0.36 0.88 6.6 3.6 9.5 85.9 89.7 2.3 2.1 5.5 0.3 0.05 0.31 0.90 9.0 3.6 7.7 87.9 89.6 2.0 2.1 5.5 0.3 0.05 0.27 0.93 11.4 4.1 5.5 89.6 91.7 1.5 2.1 4.3 0.3 0.04 0.20 0.97 16.3 4.2 3.8 91.0 92.6 0.7 2.3 4.1 0.3 0.03 0.09 1.00 24.0 1.3 13.9 84.0 88.1 3.3 2.1 5.7 0.8 0.05 0.45 0.83 6.0 1.1 11.4 86.4 89.0 2.9 2.1 5.4 0.7 0.04 0.39 0.86 7.6 0.9 8.6 89.6 91.3 2.6 2.2 3.4 0.5 0.03 0.34 0.89 10.2 | 230 242 263 269 156 183 184 234 238 240 137 154 199 200 224 130 159 184 215 | Global Journal of Researches in Engineering ( ) Volume E | |
| 600 | 0.9 | 1.1 | 7.3 90.7 92.1 2.2 2.1 3.1 0.5 0.03 0.29 0.92 12.4 | 206 | |
| 650 | 0.9 | 1.2 | 5.0 92.9 93.1 1.7 2.1 2.5 0.6 0.02 0.22 0.95 18.6 | 215 | |
| 700 | 0.9 | 1.1 | 3.9 94.1 95.5 1.1 2.0 0.9 0.6 0.01 0.14 1.00 24.1 | 225 | |
| SA | |||||
| 450 | 0.6 | 1.5 | 7.8 90.1 89.5 3.0 1.9 4.8 0.8 0.04 0.40 0.94 11.6 | 138 | |
| 500 | 0.7 | 1.6 | 6.6 91.1 90.8 3.0 1.8 7.9 0.8 0.07 0.40 0.95 13.8 | 148 | |
| 550 | 0.5 | 1.2 | 5.7 92.6 91.4 2.6 2.0 3.2 0.8 0.03 0.34 0.98 16.2 | 170 | |
| 600 | 0.5 | 1.0 | 4.8 93.7 91.4 2.1 1.9 3.8 0.8 0.03 0.28 1.00 19.5 | 187 | |
| 650 | 1.2 | 1.3 | 3.7 93.8 92.5 1.6 1.9 3.1 0.8 0.03 0.21 1.00 25.4 | 195 | |
| 700 | 0.9 | 1.3 | 3.2 94.6 92.9 1.0 1.9 3.4 0.8 0.03 0.13 1.00 29.6 | 197 | |
| © 2015 Global Journals Inc. (US) | |||||