1. I. Introduction
hin films of triple alloys consisting of the ferromagnetic metals Fe, Co and Ni have a high magnetization saturation, high magnetic permeability and low coercivity, and they are used in many areas, such as computers, read/write heads and microelectromechanical systems (MEMS). Electrochemical deposition is one of the most preferred methods for producing thin layers of alloy due to its simplicity, cost-effectiveness, versatility and relatively rapid deposition.
A characteristic feature of the electrochemical deposition of Co-Ni-Fe films is the discrepancy between the fraction of elements in the electrolyte and in the film, which makes it difficult to obtain a film with the desired electrophysical properties. This paper presents the results of a study on electrochemical deposition of a triple Co-Ni-Fe system from a chloride electrolyte solution with equivalent concentrations of Co, Ni, and Fe at 0.48, 0.083, and 0.00625 mol/l and a temperature of 70°C. The study of electrochemical deposition of Co-Ni-Fe alloys provides new insight into the mechanisms that determine the composition of the precipitated layers and their dependence on the composition of the electrolyte.
Finding the composition of the electrolyte to obtain the desired composition of the precipitated film is a very time-consuming task. The decision on the composition of the electrolyte depends on the introduction of additives into the electrolyte that improves the mechanical properties, adhesion and morphology of the films. The conducted studies of congruent electrochemical deposition of the triple Co-Ni-Fe system with equal concentrations of components in the electrolyte allow us to approach finding a solution to the electrolyte composition problem that determines the composition of precipitated films of complex composition.
2. II. Electrochemical Deposition of CO-NI-FE Films
For the deposition of Co-Ni-Fe films, a chloride electrolyte solution containing three components, CoCl 2 ?6H 2 O, FeCl 2 ?4H 2 O, and NiCl 2 ?6H 2 O, was used, with the components in a 1:1:1 molar ratio; three concentrations were tested: 0.48, 0.083, and 0.00625 mol/l [1][2][3]. Various additives were added to the electrolyte solution in the following concentrations: H 3 BO 3 -20 g/l, C 7 H 4 NaNO 3 S?2H 2 O -1.5 g/l, HCl -3 ml/l. The film from the specified electrolyte was deposited in a galvanic bath with a volume of 2 liters and a graphite anode. A vertically-oriented metallized silicon wafer was used as the cathode. The distance between the anode and the cathode was 8 cm.
Insoluble hydroxides were removed by filtration. An alloy film with a diameter of 8 cm was obtained on the metallized Ni surface of a 100 mm silicon wafer. The electrolyte was heated by a submersible heater to 70°C and mixed with a magnetic stirrer. A constant current density of 3 to 40 mA/cm 2 was maintained in the deposition area on the silicon wafer.
As the concentration of CoCl 2 , NiCl 2 , and FeCl 2 salts in the electrolyte solution increased, the resistance of the solution decreased, and this resistance determined the amount of current passing between the anode and the cathode and the voltage that changed linearly with increasing current. At a current density of 16 mA/cm 2 , the voltage between the anode and the cathode was 2 V, 3.5 V, and 5.5 V in the different electrolyte solutions.
With a difference in electrolyte concentrations of 77 times, the growth rate of Co-Ni-Fe films practically does not depend on the concentration of the electrolyte. The growth rate of Co-Ni-Fe films increases with increasing current density and half the values calculated according to Faraday's law, i.e. the cathode current output is 0.5.
1. Electrolytes with Co, Ni, and Fe concentrations of 0.48 mol/l [2] precipitated at a current density of 3.6 mA/cm 2 .
The composition of the film was Co 32 Ni 61,5 Fe 6,5 . At a low current density, the composition of the film was characterized by a high nickel content and low iron and content. When the current density of the Co-Ni-Fe film increased to 15 mA/cm 2 , the iron content rose to 41.3%, and at a current density of 20 mA/cm 2 , it remained approximately the same at 42.35%. When the current density of the Co-Ni-Fe film increased to 15 mA/cm 2 , the nickel content decreased to 7.9%, and at a current density of 20 mA/cm 2 , it remained at the same level of 7.85%. The actual change in the iron and nickel content at a current density of 10 mA/cm 2 changed the relative cobalt content to 59%. At current densities of 15 and 20 mA/cm 2 , the cobalt content had similar values of 49 and 48%. 2. Figure 2 shows the relative contents of Co, Ni, and Fe in the film during deposition from the electrolyte solution when the concentration of each component was 0.083 mol/l. There is a weak dependence on the current density at a value of more than 25 mA/cm 2 ; in the Co-Ni-Fe film, the nickel content is 19 -22%, the iron content is 28 -33%, and the cobalt content has a value of 55 -47%. With a lower current density, the composition of the Co-Ni-Fe film varies greatly; the nickel content decreases from 57 to 19%, the iron content increases from 5 to 28%, and the cobalt content ranges from 42 -55 -48%. Comparison of the dependences on the current density of the composition of films obtained from a threecomponent solution of CoCl 2 , NiCl 2 , FeCl 2 in which the concentration of the components was either 0.48 mol/l or 0.083 mol/l showed that the dependencies were similar. Increasing the current density led to a decrease in the nickel content and an increase in the iron content, and the predominant cobalt deposition persisted. Their dependence of the composition of the films on the current density was stable within a certain area.
At a current of more than 25 mA/cm 2 , the ratio of metal concentrations in the film to the metal concentrations in the electrolyte was 1.44 for cobalt, 0.97 for iron, and 0.54 for nickel, which is almost the same as the values at an electrolyte concentration of 0.48 moll/l, but closer to the concentrations in the electrolyte. The relative contents of Co, Ni, and Fe in the film differ from the composition of the electrolyte and are highly dependent on the current density. It is not possible to control the deposition with the current density to obtain a film composition equal to the composition of the electrolyte.
3. Electrochemical deposition of Co-Ni-Fe films was performed in an electrolyte solution in which the Co, Ni, Fe chloride concentrations were all 0.00625 moll/l. The solution also contained 30% hydrochloric acid (0.3 ml/l), and the temperature was 70°C. Redeposition was carried out from the same electrolyte solution, but with the addition of saccharin and boric acid, the results are shown in Table 1. The results of the study on the composition of the films are shown in Figure 3.
Without electrolyte additives the compositions of Co-Ni-Fe films shows with markers connected by lines. The Co content is indicated by rectangular markers and a dashed line. The Ni content is indicated by triangular markers and a dotted line. The Fe content is indicated by round markers and a solid line. For the samples that contained additives to the electrolyte (saccharin at concentrations of 3 g/l (at 10 mA/cm 2 ) and 1.5 g/l (at 10.7 mA/cm 2 ) and boric acid at a concentration of 20 g/l (at 11.4 mA/cm 2 )), the composition values are presented in the form of individual points. Arrows indicate changes in the composition of films for the selected current in electrolyte solutions containing the appropriate additives.
As seen in the figure, the compositions of the films depend on the current, and at a current density of 12.2 mA/cm 2 , the compositions of the metals in the Co-Ni-Fe films are close to 33% molar content of CoCl 2 , NiCl 2 , FeCl 2 salts in the electrolyte, i.e., congruent electrochemical deposition of the triple-component Co-Ni-Fe alloy is observed.
Additives to the electrolyte change the composition of the films and disrupt the congruence of deposition in various ways. Saccharin increases the content of Co and Ni and reduces the content of Fe. With a decrease in the additive from 3 to 1.5 g/l, the change in composition decreases. Boric acid at 20 g/l increases the content of Co and Fe and reduces the content of Ni.
The simultaneous addition of saccharin (1.5 g/l) and boric acid (20 ml/l) increases the content of Co, slightly reduces the content of Ni and greatly reduces the content of Fe.
Fig. 3: Dependence on the current density J in the range of 10 -12.8 mA/cm 2 percentage of the components of films C(Co; Ni; Fe), obtained from an electrolyte with an equal 33% molar content of salts CoCl 2 , NiCl 2 , FeCl 2 , each with a concentration of 0.00625 mol/l.
The thickness of the concentrated films was measured using an MSA-500 microsystem analyzer. The study of the composition of the films was carried out using a PhilipsXL 40 energy dispersion X-ray microanalyzer.
The dependences of the composition of Co-Ni-Fe films and their magnetic properties on the conditions of electrochemical deposition were studied.
Figure 4 shows the dependences of the voltage between the anode and cathode on the stabilized current densities of 5-20 mA/cm 2 at equal concentrations of CoCl 2 , NiCl 2 , and FeCl 2 salts in the electrolyte.
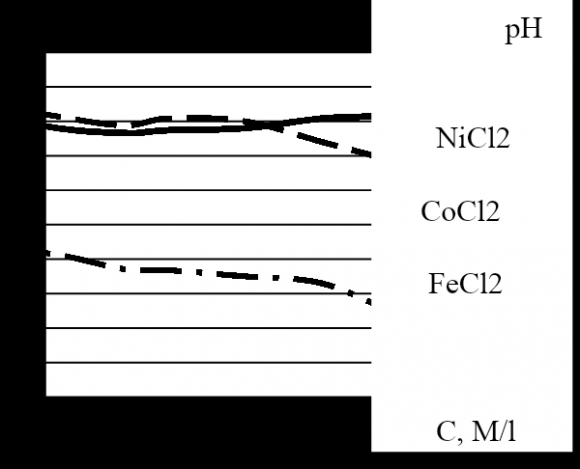
The voltage drop across the interelectrode space varies almost linearly with increasing current density from 5 to 20 mA/cm2, i.e. the ohmic conductivity of the electrolyte determines the current in the operating mode. In the galvanostatic mode of electrochemical deposition from the chloride electrolyte with equal concentrations of each component, a decrease of the concentration leads to an increase of the voltage drop across the interelectrode space due to a decrease of the amount of ions in the electrolyte. The results, which were obtained using the electrochemical deposition from the chloride electrolyte with the concentration of each component, M: 0.0074 (3); 0.08 (2); and 0.5 (1), the development of the procedure for the electrolyte preparation with the spectrum control, and the deposition at a temperature of 70°C enable one to obtain a low coercive force of Co-Ni-Fe films in a rather wide range of iron content in the film.
3. III. Magnetization and ?OERCIVE Force of Co-NI-FE Films
The magnetization and ?oercive force of the Co-Ni-Fe films were determined by the hysteresis loop of the magnetic field flux on the magnetic properties analyzer. The composition of the films on the plates was determined using an energy-dispersive X-ray microanalyzer. The results of measurements of magnetic parameters: specific magnetization B/h, coercive force Hc and composition of films of triple alloy Co-Ni-Fe are presented in Figure 6.
4. IV. Discussion of the Results
The contents of the components in the film, which were fabricated by the electrochemical deposition from the three-component solutions of FeCl 2 , CoCl 2 , and NiCl 2 with equal concentrations of the components, do not correspond to the composition of the electrolyte. At a high current density, when cobalt and iron are predominantly deposited, and a fraction of nickel in the deposit is small, the dependence of the film composition on the current density at the cathode is stabilized.
Fe-Co-Ni films were deposited by the electrochemical method from a sulfate chloride electrolyte [4], containing, moll / l: NiSO 4 ? 0.304, NiCl 2 ? 0.084, CoSO 4 0.1, FeSO 4 0.036, H 3 BO 3 20 g / l, 2 g / l stabilizer, 4 g / l tartaric acid, 4 g / l bleach, 0.1 g / l wetting agent and tartaric acid additives. Optimum conditions for obtaining high-quality films of Fe 15.6-20.6 Co 43.8-61.9 Ni 22.5-40 current density 4 A / dm 2 , temperature 40 ?, pH 2.3-3.2, tartaric acid concentration 8-12 g / l, molar Co 2+ / Ni 2+ ratio = 0.26-0.4. The composition of the film depends on the current density, electrolyte temperature, and pH. The ratio of the content of elements in the electrolyte and the film is observed -CRL for Fe, Co = 3, and for Ni = 0.5. Cobalt and iron precipitate with a concentration greater than in the electrolyte, and less nickel.
For electrochemical deposition of iron, the abnormal deposition is characteristic. Iron is deposited more intensively than cobalt and nickel. Cobalt is deposited more intensively than nickel. [4].
Estimating the deposition rate based on electrochemical potentials assumes a normal deposition of nickel, but there are many factors in the processes that determine metal deposition.
The concept of congruent electrochemical deposition determines the equality of the electrolyte and sludge compositions.
The The choice of current density used to obtain the desired composition of films is widely used in electrochemical deposition. The mechanism of the influence of the cathode current density on the composition of the resulting alloys is not defined.
One can ask the following: why does the composition of the Co-Ni-Fe film in our experiment depend on the density of the current, and at a high current density, why does the composition cease to change?
Here are some of the specifics of the processes. The resistance of the electrolyte in the electrode space is crucial for the ion conduction of dissolved salts.
The total current of the ions discharged on the cathode is much smaller than the current that is set during the process. This difference is due to the large current of ions that do not participate in deposition.
The nickel ions deposited on the cathode are formed on the anode at a high concentration and have little mobility. Iron ions are formed on the anode at a low concentration and have mobility due to the many ions of nickel. Cobalt ions are formed on the anode at a medium concentration but have greater mobility than iron atoms. These are the ions that are involved in the electrochemical reaction occurring during metal deposition. It is possible that such ions are hydroxide species formed by the hydrolysis of CoClOH, NiClOH, and FeClOH.
Current leakage is a continuous process, and the discharge of positive ions on the cathode should be accompanied by the formation of positive ions on the anode. The rate of deposition practically does not depend on the concentration of salts but depends on the density of the current, i.e., the number of positive ions created on the anode and discharged on the cathode. The mobility of active ions varies according to the different natures of the salts.
5. V. Chemical and Electrochemical
Reactions in Electrolyte a) Hydrogen index of CoCl 2 , NiCl 2 , FeCl 2 solutions In solutions of CoCl 2 , NiCl 2 , FeCl 2 , a hydrogen pH was measured using a testo 206 meter [6]. The dependence of pH on the concentration of onecomponent solutions in the concentration range of 0.01 -1 moll / l is shown in Figure 7. At a concentration of 1 moll / l, dissolving NiCl 2 gives pH = 5.8, CoCl 2 gives pH = 4.7, FeCl 2 gives pH = 2.7. The pH value characterizes the ion balance: the concentration of hydrogen, hydroxyl, acidity or alkalinity of water. Consequently, the hydrolysis of ferric chloride reduces the concentration of hydrogen during dissociation of water the most and increases the amount of hydroxyl.
A change in pH during dilution of the solution is not monotonous, but there are local peaks and a change in slope in the dependence. This means that a decrease in the concentration of the impurity is accompanied by a change in the degree of ionization and the value of the charge of ions. Local peaks are located in the dependences at different salt concentrations: NiCl 2 -0.5 moll / l; CoCl 2 -0.03 moll / l; FeCl 2 -0.02 moll / l. To obtain the same level of salt ionization, it is necessary to have solutions of each of the salts or of the order of 1 moll / l, or 0.01 moll / l. Prior to the peaks, the dependences of pH on salt concentration are linear. After peaks, there is practically no dependence of pH on the concentration of salts of NiCl 2 , CoCl 2 , but for FeCl 2 there is a change, but at a slower rate than in the linear section.
6. b) Hydrolysis Reaction and Electrode Reactions
The experimental features of electrochemical deposition are described by a sequence of chemical and electrochemical reactions. The dissolution of cobalt, nickel and iron chlorides is accompanied by a hydrolysis reaction [5].
FeCl 2 + H 2 O â??" FeClOH + H + + Cl -. NiCl 2 + H 2 O â??" NiClOH + H + + Cl -. CoCl 2 + H 2 O â??" CoClOH + H + + Cl -.On the anode, there is an electrochemical reaction, and in accordance with the size of the current and the potentials of ionization, positive ions with different concentrations are formed. At the nickel anode, chlorine dissolves the electrode when produced.
Graphite anodes are successfully used in the electrolysis of chloride salt solutions, and the anode potential at them is low. The products of the destruction of graphite anodes do not contaminate the cathode metal. On graphite anodes, chlorine is released.
Fe?? + + H + + Cl 2 ?. Ni?? + + H + + Cl 2 ?. Co?? + +H + + Cl 2 ?.Under the influence of the electric field in the electrolyte, there is a drift in the positive ions of the metal hydroxides from the anode to the cathode at a speed determined by the magnitude of the mobility of the ions and the tension of the electric field.
On the cathode, there is an electrochemical reaction of metal discharge from hydroxide species and the formation of water molecules.
FeOH + + e -+ H + = Fe + ? 2 O.NiOH + + e -+ H + = Ni + ? 2 ?.
CoOH + + e -+ H + = Co + ? 2 ?.On the cathode, there is an electrochemical reaction of metal discharge from hydroxide species and the formation of water molecules.
7. c) Concentration Polarization
The change in the electrode potential due to a change in the concentration of reagents in the electrode space during the passage of current is called concentration polarization [10]. Electrochemical reactions on the electrodes lead to a significant change in the concentrations of substances due to the slow diffusion of reagents or the removal of reaction products. The difference in the diffusion coefficients or mobility of the ions of the electrolyte component determines the balance of partial ionic currents and the content of the components in the film from the current density.
Based on the concept of concentration polarization, our results on the dependence of the concentration of the components of the triple alloy Co-Ni-Fe on the current density can be interpreted taking into account the dependence of the mobility of the ions, and not the potential of the electrode. Polarization of the electrode -the change in its potential has the same value for the ions of the three metals, and the mobility of show with a dynamic change in the potential of the electrode.
It has been experimentally established [11] that an area with an increased pH value is formed near the cathode (Figure 8). An increase in pH to a value of 7 in the region of 0.5 mm near the cathode indicates the release of water during a cathode electrochemical reaction. The greater the current density, the stronger the deviation of the composition of the electrolyte near the cathode from a uniform homogeneous one corresponding to the thermodynamic equilibrium occurs.
The ions of the electrolyte component have different diffusion coefficients during the diffusion mechanism of mass transfer and mobility values during drift of ions in an electric field, which determines the different dependence of the content of components in the film on the current density, which creates a layer of concentration polarization that limits the mass transfer of electroactive ions to the electrode. The formation of a layer with a high pH value in the rolling region of the electrolyte explains the dependence of the composition of the precipitate on the mixing of the electrolyte, which violates the layer of concentration polarization.
The thickness of the layer deposited by the electrolyte during an electrochemical reaction -SISEER is fairly large, on the order of a millimeter. A layer of this thickness has a significant effect on the mass transfer of ions to the electrodes.
The formation of a layer with a changed composition in the cathode region of the electrolyte explains the dependence of the composition of the sediment on the mixing of the electrolyte that destroys the concentration polarization layer.
The lower the concentration of the electrolyte is, the smaller the deviation of the composition of the films from the equilibrium state and the closer the composition of the films to the composition of the electrolyte. Therefore, congruent electrochemical deposition of Co-Ni-Fe alloy can be obtained only when the concentrations of the main components of the electrolyte are low and when boric acid and saccharin are not added.
The flow of ionic current disrupts the thermodynamic equilibrium in the electrolyte due to the release of the products of electrochemical reactions in the electrode regions, and the mixing of the electrolyte with mechanical or magnetic stirrers, when exposed to an ultrasonic field, during rotation or reciprocating movement of the cathode, erodes the electrode layers and evens out the distribution of salts in the electrolyte.
With a dynamic change in voltage at a speed of 10 mV / s and control of the current flowing through the electrode, the dynamic nature of the behavior of ion currents is manifested [12] (Figure 9). The presence of current peaks in the dynamic mode of voltage change is associated with the kinetic properties of the ions. The positive ions formed on the anode when approaching the cathode change the potential near the working electrode and reduce the current of the electrode. The difference in the mobility of cobalt, iron and nickel ions determines the temporal and amplitude change in the electrode current. The presence of current peaks in the dynamic mode of voltage change is associated with the kinetic properties of the ions. The positive ions formed on the anode when approaching the cathode change the potential near the working electrode and reduce the current of the electrode. The difference in the mobility of cobalt, iron and nickel ions determines the temporal and amplitude change in the electrode current.
The flow of ionic current disrupts the thermodynamic equilibrium in the electrolyte due to the release of the products of electrochemical reactions in the electrode regions, and the mixing of the electrolyte with mechanical or magnetic stirrers, when exposed to an ultrasonic field, during rotation or reciprocating movement of the cathode, erodes the electrode layers and evens out the distribution of salts in the electrolyte.
With a low cathode current density and a high concentration of salts in SISEER, a low concentration of residues of molecules arises, from which metal atoms are released and which limit the flow of ionic currents of all three metals. The composition of the film is determined by partial ion currents in accordance with the mobility of ions in the electrolyte with the initial concentration of components. The increase in current density leads to an increase in the content of molecule residues after the electrochemical reaction of metal release on the cathode. The contribution of partial currents of metal ions depends on the composition of the concentration polarization layer. There is a limitation in the deposited film of the nickel concentration alloy, which has a small amount of mobility. Iron-containing ions have more mobility in the concentration polarization layer. The concentration of iron in the film increases. The mobility of cobaltcontaining ions in SISEER is high, which leads to a strong increase in the relative content of cobalt in the film.
Stirring the salt solution disrupts SISEER. Carrying out the process of electrochemical precipitation without mixing reduces the nickel content and increases the iron content in the film, since there is no violation of CISER. With a high cathode current density and a low concentration of salts in the electrolyte region near the cathode, the flow of ionic currents of all three metals is not limited. As a result, congruent deposition of the Co-Ni-Fe alloy film is observed.
Establishes [13] that at a given speed of rotation of the cathode, it is possible to select the density of the deposition current to obtain a given film composition at a different ratio of nickel and iron in the electrolyte. Electrochemical precipitation of Ni-Fe alloys with a wide spectrum of composition was carried out from a sulfate-chloride electrolyte containing Ni 2+ / Fe 2+ ions (nickel sulfate -iron chloride) in proportions of 5: 1, 10: 1, 15: 1, 20: 1, 25: 1, boric and ascorbic acids, saccharin, sodium lauryl sulfate (Figure 10). The processes were carried out at current densities (20 -160 mA/cm2), room temperature 23oC, pH = 3.0. The cathode was a platinum disk moving at a speed of 0.0005 -0.008 cm / s. The change in cathode speed and current density made it possible, as shown in Figure 10, for a given ratio of nickel and iron 10: 1, 15: 1, 20: 1, 25: 1 in the electrolyte to obtain the composition of the permalloy film Ni 81 Fe 19 .
In our experiment with electrolyte stirring, according to the terminology adopted in the book [14], the effect of diffusion on the rate of electrochemical processes is not basic. The transport of electroactive ions is determined by the migration of ions at a high current of the background electrolyte. It should be noted that in the book it is believed that the voltage drop in the electrolyte occurs near the electrodes, and the field strength in the electrolyte is very small, which contradicts the continuity of the current.
Based on the experimental results of obtaining Co-Ni-Fe films, a mechanism of electrochemical deposition based on the phenomena of salt hydrolysis, discharge of positive ions at the anode, drift of ions in the volume of the electrolyte and discharge of negative ions at the cathode with precipitation of metals and the formation of SISEER [15], has been proposed.
Experimental data show a difference between the deposition rate of the three components of the Co-Ni-Fe alloy and the metal content in the electrolyte. The determining factor is the equality of the charges of the ions that form the partial currents of the electroactive ions. For the Triple Alloy Co-Ni-Fe, such conditions are met at an electrolyte component concentration of 0.06 M/L and a current density of 12 mA/cm 2 , which gives congruent deposition.
8. VI. Conclusion
The deposition of Co-Ni-Fe films was performed in a chloride electrolyte with a ratio of C Co : C Ni : C Fe = 1: 1: 1, and technology for preparing an electrolyte via filtration and performing deposition at a temperature of 70°C was developed. It was found that the desired 1:1:1 ratio of concentrations of the metals in the film was achieved at a current density of 12.5 mA/cm 2 and a metal chloride concentration of 0.00625 M/L. Co-Ni-Fe films are obtained reproducibly with minimal mechanical stresses and with good adhesion to the nickel sublayer during electrochemical deposition. The mechanistic study of the electrochemical deposition of a Co-Ni-Fe alloy as the electrolyte concentration changes resulted in congruent deposition of the three-component alloy. The use of congruent electrochemical deposition will simplify the choice of electrolyte composition for obtaining films with a complex composition. A review of the literature shows that none of the researchers used this method of research.
| H and composition of Co-Ni-Fe films after electrochemical deposition from an electrolyte solution | ||||||||||
| in which each component had a concentration of 0.00625 mol/l. The solution also contained added boric acid and | ||||||||||
| saccharin. Process parameters: pH of the electrolyte, voltage between the anode and cathode U, electric current I, | ||||||||||
| deposition time t, film growth rate V. | ||||||||||
| # Ph | U, V I, Ma T, Min H, µ V, Nm/Min Co, % Ni, % Fe, % Electrolyte Additive | |||||||||
| 1 | 2,62 6,8 | 500 | 30 | 4,34 | 145 | 42 | 13,5 | 44 | ||
| 2 | 2,25 5,8 | 500 | 30 | 3,19 | 106 | 34 | 35 | 31 | Saccharin 3 g/l | |
| 3 | 2,8 | 8,1 | 570 | 30 | 4,36 | 177 | 51 | 20 | 30 | |
| 4 | 2,2 | 8,55 | 570 | 30 | 5,31 | 145 | 45,3 | 17,8 | 36,9 | boric acid 20 ml/l |
| 5 | 2,35 7,75 | 534 | 30 | 4,5 | 150 | 44,5 | 24 | 31,5 | Saccharin 1,5 g/l | |
| 6 | 2,7 | 10,4 | 610 | 10 | 1,9 | 190 | 35,9 | 29,7 | 33,6 | |
| 7 | 2,55 8,3 | 610 | 10 | 2,8 | 88 | 50,6 | 13,7 | 35,7 | Saccharin 1,5 g/l boric acid 20 ml/l | |